Share This Page
Drug Price Trends for DAYSEE
✉ Email this page to a colleague
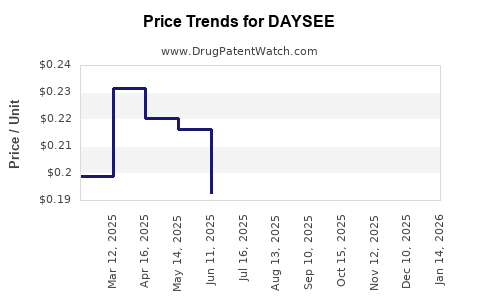
Average Pharmacy Cost for DAYSEE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| DAYSEE 0.15-0.03-0.01 MG TAB | 68180-0846-11 | 0.12125 | EACH | 2025-11-19 |
| DAYSEE 0.15-0.03-0.01 MG TAB | 68180-0846-13 | 0.12125 | EACH | 2025-11-19 |
| DAYSEE 0.15-0.03-0.01 MG TAB | 68180-0846-11 | 0.15276 | EACH | 2025-10-22 |
| DAYSEE 0.15-0.03-0.01 MG TAB | 68180-0846-13 | 0.15276 | EACH | 2025-10-22 |
| DAYSEE 0.15-0.03-0.01 MG TAB | 68180-0846-13 | 0.18248 | EACH | 2025-09-17 |
| DAYSEE 0.15-0.03-0.01 MG TAB | 68180-0846-11 | 0.18248 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for DAYSEE
Introduction
DAYSEE (generic: Amitriptyline) is a widely prescribed tricyclic antidepressant primarily used for depression, neuropathic pain, and migraine prophylaxis. Its established clinical efficacy, affordability, and extensive market penetration make it a significant focus within the pharmaceutical landscape. This analysis evaluates the current market environment, competitive positioning, regulatory factors, and projects future pricing trends for DAYSEE over the next five years.
Current Market Landscape
Global Market Overview
The global antidepressant market, valued at approximately USD 17 billion in 2022, continues to expand driven by increased recognition of mental health issues and broader off-label uses for older medications like DAYSEE. The prevalence of depression affects over 280 million individuals worldwide, constituting a persistent demand driver (WHO, 2021). Importantly, generic forms of amitriptyline account for a significant proportion of prescriptions, given their affordability and long-standing clinical acceptance.
Market Penetration & Usage
DAYSEE is predominantly utilized in developed markets such as North America and Europe, where healthcare infrastructure supports widespread prescribing. The drug’s usage extends into developing regions, fueled by generic availability and cost-effectiveness. However, newer antidepressants like SSRIs and SNRIs have encroached upon its market share due to favorable side effect profiles—yet, DAYSEE remains relevant because of its low cost and efficacy in specific indications.
Competitive Environment
The drug faces competition from multiple generic manufacturers and branded alternatives. Key competitors include Pfizer’s amitriptyline formulations, Teva Pharmaceuticals, Mylan, and other regional generic producers. Patent-related exclusivities are largely expired, fostering an intensely competitive landscape that suppresses price levels.
Regulatory and Market Dynamics
Regulatory bodies such as the FDA and EMA have approved DAYSEE since the 1960s. However, concerns over anticholinergic side effects and cardiovascular risks impact prescribing trends, especially among elderly populations. Recent initiatives emphasizing mental health and chronic pain management could influence demand, but safety concerns remain a limiting factor.
Price Analysis and Historical Trends
Pricing Overview
Historically, the pricing of DAYSEE has been characterized by low per-unit costs, reflecting its generic status. Average wholesale acquisition costs (AWAC) in the US stabilize around USD 0.10–0.20 per tablet, with retail prices slightly higher due to distribution margins.
Factors Influencing Price Stability
- Generic Market Saturation: High competition extorts downward pressure on prices.
- Manufacturing Costs: Remain relatively stable due to simple synthesis routes.
- Regulatory and Quality Standards: Minimal impact on cost, given longstanding approval status.
- Demand Stability: Consistent, driven by chronic conditions and certain off-label uses.
Price Fluctuations and Anomalies
While generally stable, localized price variations are observed due to healthcare policy changes, reimbursement adjustments, or supply chain disruptions. Notably, during the COVID-19 pandemic, supply chain instability caused temporary price fluctuations in some regions.
Future Price Projections (2023–2028)
Assumptions and Methodology
Projections utilize a combination of historical price data, market growth estimates, regulatory outlooks, and competitive dynamics. A conservative decline in unit prices is envisaged to persist owing to intensifying generic competition, with potential stabilization as demand plateaus or slightly increases due to expanding indications.
Projected Trends
- Short-term (2023–2024): Prices expected to decline modestly by 5–8% annually, driven by existing market saturation and pricing pressure.
- Mid-term (2025–2026): Potential stabilization as market adjustments occur; minor fluctuations possible contingent on new formulary placements.
- Long-term (2027–2028): Slight price stabilization or marginal increase if new off-label indications or formulations are introduced, or if manufacturing costs diminish due to technological advancements.
Influence of Market & Regulatory Shifts
Emerging safety concerns could influence prescriber preferences away from DAYSEE, leading to reduced demand, which might further depress prices or prompt manufacturers to modify formulations. Conversely, increased emphasis on cost-effective treatments within healthcare budgets could sustain or slightly elevate demand, providing price resilience.
Regional Variations and Market Opportunities
- North America: Price decline driven by fierce generic competition, with average tablet prices expected to fall below USD 0.10 by 2028.
- Europe: Slightly higher prices due to regional regulations and health policies, with stabilization near current levels.
- Emerging Markets: Lower prices due to increased import competition, though demand is growing owing to affordability and healthcare expansion.
Market Growth Drivers and Constraints
- Growth Drivers: Chronic pain management needs, mental health awareness, and increasing approval for off-label uses.
- Constraints: Safety profile limitations, competition from newer classes, and regulatory restrictions for elder use.
Conclusion
DAYSEE maintains a robust position within the global antidepressant market primarily due to its cost efficiency and established clinical efficacy. While current prices are low and under sustained downward pressure, the outlook suggests minimal fluctuation in the coming years, with potential stabilization or slight increases influenced by regulatory and market dynamics. Pharmaceutical stakeholders must monitor safety concerns and evolving prescribing practices that could impact demand and, consequently, pricing strategies.
Key Takeaways
- Dominance Through Cost-Effectiveness: The low price point of DAYSEE is central to its sustained prescribing, especially in cost-sensitive markets.
- Competitive Intensity Limits Price Growth: Extensive generic competition exerts continuous downward pressure.
- Demand Stability Ensures Revenue Base: Consistent use in depression and chronic pain management supports stable demand despite competition.
- Safety and Regulatory Factors Play Critical Roles: Emerging safety concerns could influence prescribing patterns and affect future pricing.
- Market Expansion Opportunities: Growing mental health awareness presents long-term prospects, particularly in emerging markets.
FAQs
-
What factors primarily influence the price of DAYSEE globally?
Market competition, manufacturing costs, regulatory environment, demand stability, and regional healthcare policies profoundly impact its pricing. -
Will DAYSEE's price increase due to new medical indications?
Potentially, if expanded indications lead to higher demand or formulation modifications, prices could stabilize or rise slightly, though competition may still keep prices low. -
How does safety concerns about DAYSEE affect its market value?
Safety issues can reduce prescribing rates among certain populations, decreasing demand and exerting downward pressure on prices. -
What are the key regional differences in DAYSEE pricing?
Developed markets like North America and Europe experience more price competition and lower prices, while emerging markets may have higher prices due to import costs and regulatory factors. -
How might regulatory changes impact DAYSEE’s future market?
Stricter regulations regarding safety and prescribing might limit its use, whereas policies promoting affordable mental health treatments may bolster demand.
Sources
[1] World Health Organization. (2021). Depression and Other Common Mental Disorders: Global Health Estimates.
[2] MarketWatch. (2022). Global Antidepressant Market Size, Share & Trends.
[3] U.S. Food and Drug Administration. (FDA). Medical Review Data for Amitriptyline.
[4] Pharmaceutical Commerce. (2022). Price Trends of Generic Psychotropics.
More… ↓
