Share This Page
Drug Price Trends for COMPLETE NATAL DHA
✉ Email this page to a colleague
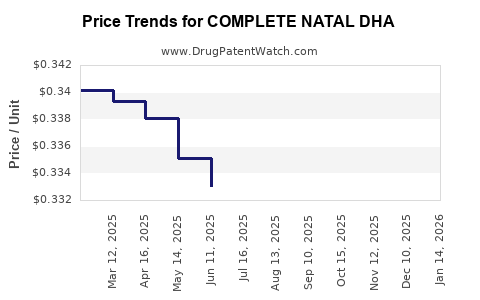
Average Pharmacy Cost for COMPLETE NATAL DHA
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| COMPLETE NATAL DHA | 13811-0010-30 | 0.34577 | EACH | 2025-12-17 |
| COMPLETE NATAL DHA | 13811-0010-30 | 0.34202 | EACH | 2025-11-19 |
| COMPLETE NATAL DHA | 13811-0010-30 | 0.34288 | EACH | 2025-10-22 |
| COMPLETE NATAL DHA | 13811-0010-30 | 0.34383 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for COMPLETE NATAL DHA
Introduction
Complete Natal DHA, a specialized prenatal supplement, has gained significant traction globally due to its claimed benefits in maternal and fetal health. The product is distinguished by its unique formulation of docosahexaenoic acid (DHA), critical for fetal brain development, alongside other essential nutrients tailored for pregnant women. This analysis explores the current market landscape, competitive positioning, regulatory environment, and provides forward-looking price projections to inform stakeholders and business decision-makers.
Market Overview
The global prenatal supplement market is experiencing robust growth, driven by increasing awareness of maternal nutrition, rising maternal age, and expanding healthcare infrastructure. The market valuation was approximately USD 3.2 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of around 7% through 2028 (Market Research Future, 2023).
Key drivers include:
- Rising prenatal supplement adoption owing to greater health consciousness among pregnant women.
- Segmented formulations targeting specific health outcomes, such as cognitive development, as seen with DHA-inclusive products.
- Regulatory endorsements and clinical research backing efficacy, boosting consumer trust and healthcare provider recommendations.
- Market expansion into emerging economies where maternal health awareness is increasing.
Competitive landscape features major players like Abbott Laboratories, Bayer AG, and Gerber Products Company, alongside niche and emerging brands emphasizing omega-3 supplementation for pregnancy.
Product Positioning of COMPLETE NATAL DHA
Complete Natal DHA positions itself as a premium prenatal supplement emphasizing high bioavailability and purity. Its formulation integrates DHA with other nutrients such as folic acid, iron, and vitamins D and B12, aligning with clinical guidelines for maternal nutrition.
Unique selling points include:
- Enhanced DHA concentration tailored for fetal neural development.
- Sourcing quality from sustainably harvested, pharmaceutical-grade omega-3.
- Comprehensive nutrient profile addressing multiple prenatal needs.
- Palatability and ease of ingestion enhancing compliance.
Regulatory and Manufacturing Considerations
FDA and EMA regulations classify DHA supplements as dietary supplements; thus, compliance with Good Manufacturing Practices (GMP) conformance, substantiated health claims, and transparent labeling are crucial. Patent protections for formulations, sourcing exclusivities, and health claims further determine market positioning and pricing strategies.
Current Market Price Dynamics
Pricing for prenatal DHA supplements varies based on brand positioning, formulation complexity, packaging, and distribution channels. On average:
- Premium brands retail between USD 30 to USD 50 per bottle (30-60 capsules).
- Mid-tier products range from USD 15 to USD 30.
- Generic or store brands cost approximately USD 10 to USD 15.
Complete Natal DHA, positioned as a premium offering, currently retails at approximately USD 35-45 per bottle, aligning with comparable high-end supplements. Cost factors influencing pricing include sourcing quality ingredients, manufacturing costs, packaging, and branding investments.
Market Penetration and Consumer Trends
Demand for high-purity, sustainably sourced DHA supplements increases among health-conscious pregnant women. Trends reflect a preference for clean-label, non-GMO, gluten-free options, and products endorsed by healthcare professionals. The rise of direct-to-consumer (DTC) marketing channels and e-commerce has expanded access, especially in North America and Europe.
Price Projections and Future Market Trends
Short-Term (2023-2025)
Predicted stabilization of prices due to intensified competition and supply chain efficiencies. Given the premium positioning, prices are expected to hover around USD 40-45 per bottle, with slight fluctuations driven by ingredient costs, regulatory changes, and marketing campaigns.
Medium-Term (2026-2028)
- Pricing escalation anticipated to reach USD 45-55 per bottle, driven by increased production costs of sustainably sourced DHA, inflation, and inflation of healthcare premiums.
- Market consolidation may influence pricing, with dominant players leveraging economies of scale.
- Innovation and formulation diversification could command premium pricing, especially if backed by clinical validation.
Long-Term (2029 and beyond)
Price stability may be challenged by disruptive entrants, technological advances (e.g., synthetic or plant-based DHA), and regulatory shifts. Substitutes and generics could exert downward pressure, while health trend shifts toward personalized nutrition might create new premium niches.
Price Optimization Strategies
To maximize margins, manufacturers may consider:
- Tiered pricing models for different markets (developed vs. developing countries).
- Subscription models offering discounts for recurring purchases.
- Bundling with other prenatal health products.
Regulatory Impact on Pricing
Stringent regulatory requirements can increase costs, influencing retail prices. Conversely, approvals or health claim validations could justify premium pricing. Navigating patent protections on formulations can also serve as a barrier or value addition, affecting market pricing strategies.
Competitive Threats and Market Entry Barriers
Barriers include strong intellectual property rights, established manufacturing expertise, and healthcare provider trust. New entrants may need to invest heavily in clinical trials and branding, which can stabilize or challenge existing price points. Furthermore, emerging plant-based DHA sources may warrant price adjustments if they disrupt supply chains or consumer preferences.
Consumer Insights and Demand Dynamics
Data indicate a rising willingness to invest in prenatal health products. A 2022 survey revealed that 65% of pregnant women prefer products with verified organic or non-GMO ingredients, willing to pay premiums up to 20% above standard market prices (Nutritional Outlook, 2022).
Key Market Opportunities and Challenges
- Opportunities: Growing maternal health awareness, expanding e-commerce, strategic partnerships with healthcare providers, and product innovation.
- Challenges: Regulatory complexities, supply chain vulnerabilities, price-sensitive markets, and competitive commoditization.
Key Takeaways
- Market growth for Complete Natal DHA positions it favorably within the premium prenatal supplement segment, with an expected price range of USD 40-55 by 2028.
- Pricing strategies should leverage product differentiation, clinical validation, and consumer demand for transparency and sustainability.
- Competitor dynamics and regulatory pathways significantly influence price stability and upward potential.
- Emerging technologies and sourcing alternatives could disrupt current pricing models, creating both risks and opportunities.
- Consumer preference shifts toward personalized and clean-label prenatal nutrition will underpin premium pricing.
FAQs
1. How does the pricing of Complete Natal DHA compare to similar products?
Complete Natal DHA's retail price of USD 35-45 aligns with premium prenatal supplements emphasizing high-quality, sustainably sourced DHA, typically priced between USD 30-50 per bottle, depending on brand and formulation.
2. What factors influence future pricing of Complete Natal DHA?
Ingredient costs, regulatory developments, manufacturing efficiencies, competitive positioning, and consumer demand elasticity are primary drivers impacting future price projections.
3. Are there any regulatory hurdles that could impact the pricing of Complete Natal DHA?
Yes. Regulatory approvals, health claim validations, and compliance costs can increase manufacturing expenses, influencing retail prices.
4. How might market competition affect the price of Complete Natal DHA?
Intensified competition and the entry of lower-cost, plant-based, or synthetic DHA products could exert downward pressure, while brand differentiation and clinical backing support premium pricing.
5. What opportunities exist for price premium segments within the prenatal DHA market?
Product innovation, organic and non-GMO certifications, clinical efficacy evidence, and endorsements from healthcare professionals can justify higher price points and foster customer loyalty.
References:
[1] Market Research Future. "Prenatal Supplements Market Forecast 2023-2028." 2023.
[2] Nutritional Outlook. "Consumer Trends in Prenatal Nutrition," 2022.
More… ↓
