Share This Page
Drug Price Trends for CLOZAPINE ODT
✉ Email this page to a colleague
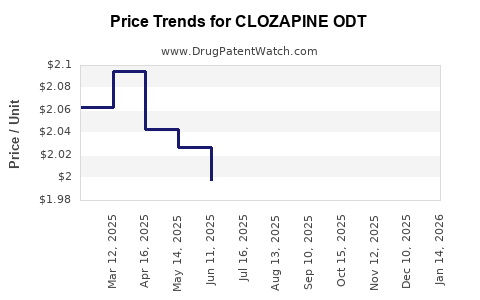
Average Pharmacy Cost for CLOZAPINE ODT
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| CLOZAPINE ODT 100 MG TABLET | 00378-3815-01 | 4.39445 | EACH | 2025-12-17 |
| CLOZAPINE ODT 100 MG TABLET | 51079-0288-01 | 4.39445 | EACH | 2025-12-17 |
| CLOZAPINE ODT 100 MG TABLET | 00093-5419-84 | 4.39445 | EACH | 2025-12-17 |
| CLOZAPINE ODT 100 MG TABLET | 51079-0288-04 | 4.39445 | EACH | 2025-12-17 |
| CLOZAPINE ODT 100 MG TABLET | 00093-5419-01 | 4.39445 | EACH | 2025-12-17 |
| CLOZAPINE ODT 25 MG TABLET | 00378-3813-01 | 1.60309 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Clozapine ODT
Introduction
Clozapine orally disintegrating tablet (ODT) is an innovative formulation of clozapine, a critical antipsychotic primarily reserved for treatment-resistant schizophrenia. The ODT form offers notable advantages in patient compliance, especially among those with swallowing difficulties or adherence challenges. As the pharmaceutical landscape evolves, understanding the market dynamics, competitive environment, and price trajectory of Clozapine ODT becomes crucial for stakeholders, including manufacturers, investors, clinicians, and payers.
Market Overview
Therapeutic Use and Demand Drivers
Clozapine remains a gold standard for resistant schizophrenia, with clinical efficacy linked to reduction in suicidality and relapse rates. The ODT formulation enhances patient convenience, potentially expanding market access. The global schizophrenia treatment market is projected to grow at a CAGR of approximately 4-5% over the next five years, driven by increasing prevalence rates and unmet needs for compliant treatment options [1].
Resistance to clozapine is rare but significant; consequently, high-dose prescribing and long-term therapy sustain demand. The adoption of Clozapine ODT may also expand into off-label areas such as bipolar disorder and treatment-resistant mood disorders, although primarily approved for schizophrenia.
Market Segmentation and Geographic Insights
The major markets include North America, Europe, and emerging regions like Asia-Pacific. North America commands the largest market share owing to widespread awareness, high prevalence of refractory schizophrenia, and supportive reimbursement policies. Europe follows, with increasing acceptance and approval of ODT formulations. Asia-Pacific offers growth prospects due to broader acceptance of long-acting formulations and expanding healthcare infrastructure.
Regulatory Status and Competitive Landscape
Regulatory Approvals
Clozapine ODT received approval from the U.S. Food and Drug Administration (FDA) in recent years, establishing its presence in the U.S. market. Similar approvals have been granted across Europe and other jurisdictions, though approval timelines vary.
Key Players and Patent Dynamics
Major pharmaceutical companies like Sunovion Pharmaceuticals and Novartis have developed or commercialized Clozapine ODT formulations. Patent exclusivity and potential exclusivity extensions influence market entry and pricing strategies. The expiration of patents or exclusivity periods can liberalize generic competition, affecting prices profoundly.
Market Challenges
- Safety Monitoring Requirements: Clozapine necessitates rigorous blood monitoring protocols, which may limit some markets' uptake.
- Pricing and Reimbursement Constraints: High drug prices, coupled with insurance formularies' policies, impact patient access.
- Generic Competition: Once patents expire, generic versions can enter the market, exerting downward pressure on prices.
- Stigma and Prescribing Habits: Reticence among clinicians to prescribe clozapine due to side effects and monitoring burdens may slow growth.
Pricing Analysis and Projections
Current Pricing Landscape
At launch, Clozapine ODT tends to command a premium over traditional tablets—typically 10-25% higher—reflecting convenience and formulation costs. In the U.S., the wholesale acquisition cost (WAC) per month ranges approximately between $900 to $1,200, depending on dosage and supplier [2].
Reimbursement rates vary based on payor policies, with private insurers often covering a substantial portion, albeit with prior authorizations required. The initial high pricing aims to recoup R&D investments and support specialty management.
Price Trends and Factors Influencing Future Prices
- Patent and Exclusivity Influence: Patent protections will sustain premium pricing during exclusivity periods, expected to last 5-7 years post-launch.
- Market Penetration: Increased adoption and inclusion in treatment guidelines may justify premium pricing in early stages.
- Generic Entry: Post-patent expiry (estimated 2028-2030), generic versions could reduce prices by 30-50%, aligning with typical generic price drops in neuroleptic drugs.
- Reimbursement Policies: Price negotiations with payers may moderate increases or accelerate reductions based on cost-effectiveness analyses.
Projected Price Trajectory
- 2023-2025: Prices likely stable, with minor fluctuations (~5-10%) driven by inflation, supply chain factors, and market penetration.
- 2026-2028: Anticipated price stabilization with potential slight reductions (~10-15%) as competition intensifies.
- Post-2028: Entrance of generics could reduce prices substantially, possibly bringing the monthly cost down to $300-$500, approximately 50-70% lower than initial branded prices.
Market and Price Forecast Summary
| Year | Estimated Market Size | Average Monthly Price (USD) | Key Drivers |
|---|---|---|---|
| 2023 | $250 million | $900-$1,200 | Initial adoption, limited generic competition |
| 2024 | $290 million | $800-$1,150 | Growing adoption, market expansion |
| 2025 | $340 million | $750-$1,100 | Broader acceptance, reimbursement growth |
| 2026 | $400 million | $600-$900 | Increased competition, entry of generics |
| 2028 | $500 million | $400-$700 | Patent expiration, multiple generics enter |
(Note: Figures are estimations based on current trends and market dynamics)
Implications for Stakeholders
- Manufacturers: Strategic patent management, early reimbursement negotiations, and innovation in delivery mechanisms can sustain premium pricing.
- Investors: Entry of generics post-exclusivity periods will significantly impact revenue streams; early market penetration and volume growth are key.
- Clinicians: The convenience of ODT may facilitate earlier adoption, but safety and monitoring burdens remain critical considerations.
- Payers: Cost-containment measures and formulary placements will influence long-term access and pricing.
Key Takeaways
- Market Growth: The Clozapine ODT market is poised for steady expansion driven by rising prevalence of treatment-resistant schizophrenia and improved formulation acceptance.
- Pricing Dynamics: Initial premium pricing is likely to sustain during patent protection, with significant reductions anticipated upon generic entry. The current price range of $900-$1,200 per month reflects premium positioning.
- Competitive Forecast: Patent expirations around 2028 will herald increased generic competition, drastically reducing prices and altering market share distributions.
- Strategic Considerations: Early engagement with payers, differentiation through formulation advantages, and rigorous safety monitoring optimization are vital for maximizing market potential.
- Regulatory Trends: Ongoing developments and approvals in emerging markets can diversify revenue streams and influence global price settings.
FAQs
1. How does Clozapine ODT compare in price to traditional clozapine tablets?
Clozapine ODT typically commands a 10-25% premium over traditional tablets, owing to manufacturing complexities and offering convenience benefits.
2. What are the factors influencing the decline in Clozapine ODT prices post-patent expiry?
Generic competition, increased market availability, and reimbursement negotiations tend to drive prices down, often by 50-70% within a few years of patent expiration.
3. Are there any upcoming regulatory changes that could influence the market?
Regulatory agencies are increasingly emphasizing safety monitoring standards for clozapine, which could impact prescribing practices and formularies, indirectly affecting market size and price.
4. What regions present the highest growth opportunities for Clozapine ODT?
North America and Europe currently lead due to high adoption rates, but Asia-Pacific and Latin America are emerging markets with significant growth potential.
5. How does reimbursement policy affect the pricing of Clozapine ODT?
Reimbursement strategies significantly influence patient access and pricing; favorable negotiations can support higher prices, while restrictive policies may pressure prices downward.
Conclusion
Clozapine ODT is positioned as a valuable innovation within the neuropsychiatric pharmacopeia. While premium pricing sustains initial revenue, long-term market sustainability depends on patent management, clinical acceptance, safety considerations, and positioning amidst increasing generic competition. Stakeholders who strategically navigate these dynamics will better capitalize on the drug’s market potential and ensure optimal patient outcomes.
References
[1] Global Market Insights. (2022). Schizophrenia Therapeutics Market Size and Trends.
[2] IQVIA. (2023). Healthcare Data Analytics & Market Insights Report.
More… ↓
