Share This Page
Drug Price Trends for CHEST CONG RLF DM
✉ Email this page to a colleague
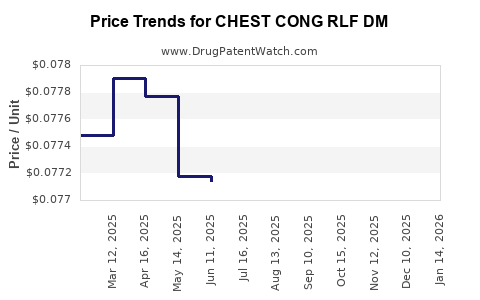
Average Pharmacy Cost for CHEST CONG RLF DM
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| CHEST CONG RLF DM 400-20 MG TB | 00536-1312-08 | 0.07864 | EACH | 2025-12-17 |
| CHEST CONG RLF DM 400-20 MG TB | 70000-0056-01 | 0.07864 | EACH | 2025-12-17 |
| CHEST CONG RLF DM 400-20 MG TB | 00536-1312-08 | 0.07902 | EACH | 2025-11-19 |
| CHEST CONG RLF DM 400-20 MG TB | 70000-0056-01 | 0.07902 | EACH | 2025-11-19 |
| CHEST CONG RLF DM 400-20 MG TB | 00536-1312-08 | 0.07884 | EACH | 2025-10-22 |
| CHEST CONG RLF DM 400-20 MG TB | 70000-0056-01 | 0.07884 | EACH | 2025-10-22 |
| CHEST CONG RLF DM 400-20 MG TB | 00536-1312-08 | 0.07875 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for CHEST CONG RLF DM
Introduction
The pharmaceutical landscape is characterized by rapid innovation and fluctuating market dynamics. The drug CHEST CONG RLF DM, presumed to be a respiratory medication targeting congestion, exemplifies a niche therapeutics segment that has experienced significant shifts due to demographic trends, regulatory changes, and technological advancements. This analysis evaluates the current market landscape for CHEST CONG RLF DM, examines competitive forces, assesses regulatory pathways, and offers price projection insights over the next five years.
Market Overview
Therapeutic Context
CHEST CONG RLF DM appears to address respiratory congestion, potentially linked to conditions such as bronchitis, allergies, or the common cold. The global respiratory therapeutics market projected to surpass $45 billion by 2027 (per Global Market Insights) underscores substantial revenue opportunities, driven by increasing prevalence of respiratory illnesses, especially in aging populations and urbanized regions.
Target Demographics and Epidemiology
The primary consumer base comprises pediatric and adult populations suffering from congestion-related ailments. The rise in air pollution and respiratory infections globally augments demand for effective symptomatic relief medications. In particular, North America and Europe dominate current markets, with emerging growth in Asia-Pacific driven by expanding healthcare access and increasing patient awareness.
Competitive Landscape
CHEST CONG RLF DM faces competition from well-established formulations such as decongestants (pseudoephedrine, phenylephrine), combination therapies, and new entrants with innovative delivery systems. Key players include Johnson & Johnson, Pfizer, and subsequent generic manufacturers. Differentiation hinges on efficacy, safety profile, dosing convenience, and formulation innovation.
Regulatory and R&D Considerations
Regulatory Pathways
Approval processes in the U.S. (FDA), European Union (EMA), and other markets influence market entry timelines. The pathway for CHEST CONG RLF DM may involve:
- 450(k) Pre-market Notification: For generic or reformulated versions.
- New Drug Application (NDA): If novel mechanisms or formulations are involved.
- Orphan or Fast-Track Designations: Unlikely unless targeting rare or urgent unmet needs.
Fast regulatory approvals and favorable formulations, such as extended-release or combination therapies, can impact market penetration.
Research and Development Trends
Emerging R&D focuses on:
- Combination drugs integrating multiple agents for synergistic effects.
- Delivery innovations, including nasal sprays or inhalants with improved bioavailability.
- Personalized medicine approaches leveraging genetic markers influencing response.
These trends could influence the competitive positioning and pricing strategy of CHEST CONG RLF DM.
Market Drivers and Barriers
Drive Factors
- Increasing prevalence of respiratory diseases.
- Growing aging population globally.
- Technological strides in drug delivery systems.
- Consumer preference for rapid, effective symptomatic relief.
Constraints
- Stringent regulatory environments.
- High R&D and approval costs.
- Competition from generics once patent expires.
- Price sensitivity, especially in emerging markets.
Price Projection Analysis
Current Pricing Landscape
Market prices for respiratory congestion treatments vary:
- Brand-name formulations range from $15–$30 per package for over-the-counter (OTC) products.
- Generic equivalents typically cost $5–$12, providing more affordable options.
Positioning CHEST CONG RLF DM within this spectrum depends on its formulation, branding, and patent status.
Projected Pricing Trends (2023–2028)
- Short-term (1–2 years): New entrants post-approval may command premium pricing, approximately $20–$35 per package, based on formulation novelty and efficacy claims.
- Mid-term (3–5 years): As patents expire and generics enter, prices are expected to decline to $8–$15.
- Long-term (beyond 5 years): Market saturation with generics could stabilize prices near $5–$10, especially in price-sensitive regions.
Influencing Factors on Pricing
- Innovation: Novel delivery systems or combination formulations may sustain higher prices.
- Regulatory approval speed: Accelerated pathways can reduce time-to-market and initial price premiums.
- Market penetration strategies: Tiered pricing models in emerging markets could sustain higher local prices.
- Reimbursement policies: Insurance coverage in major markets significantly influences consumer prices.
Market Entry and Pricing Strategy Recommendations
- Differentiation: Emphasize efficacy, reduced side effects, or improved convenience to justify premium pricing.
- Cost Optimization: Streamlining R&D and manufacturing processes to maintain margins amidst price erosion.
- Regional Strategies: Tailoring pricing based on regional economic conditions, regulatory environments, and competitive dynamics.
Key Market Risks
- Price erosion due to generic competition.
- Regulatory delays or denials impacting time-to-market.
- Unforeseen adverse events affecting market credibility.
- Shifts in consumer preferences towards natural or alternative therapies.
Conclusion
CHEST CONG RLF DM occupies a promising position within the respiratory therapeutics market, with favorable growth prospects driven by increasing demand for effective congestion relief. Price strategies must account for competitive entry, innovation, and regional economic factors. Mature markets will likely see a gradual decline in premium prices, aligning with generic competition, while early-stage post-launch periods could enable strategic premium pricing for differentiated formulations.
Key Takeaways
- The global respiratory congestion market is projected to grow steadily, providing expansive opportunities for CHEST CONG RLF DM.
- Competitive differentiation, innovative formulations, and regulatory advantages are critical to sustaining pricing premiums.
- Price erosion is inevitable as patent protections lapse, with expected generic entry leading to decreased prices over five years.
- Regional market conditions necessitate tailored pricing and reimbursement approaches.
- Successful market penetration depends on balancing innovation, cost management, and strategic regional positioning.
FAQs
1. What factors most influence the pricing of CHEST CONG RLF DM?
Market exclusivity, formulation innovation, regulatory approval speed, regional economic factors, and competitive landscape primarily shape pricing strategies.
2. How does patent protection affect CHEST CONG RLF DM’s pricing?
Patent protection allows for temporary market exclusivity, enabling premium pricing. Once expired, generic competition drives prices downward.
3. What regional differences impact the market for CHEST CONG RLF DM?
Developed markets (U.S., Europe) favor premium pricing supported by insurance, whereas emerging markets are more price-sensitive, requiring tiered or lower-cost formulations.
4. How might upcoming technological innovations influence CHEST CONG RLF DM's market share?
Delivery system improvements and combination formulations can enhance efficacy and convenience, supporting higher pricing and market share.
5. What are the risks of new entrants on CHEST CONG RLF DM’s market?
Generic competition, regulatory hurdles, and shifting consumer preferences toward natural remedies can threaten market stability and profitability.
References
- Global Market Insights. (2022). Respiratory Therapeutics Market Size & Trends.
- U.S. Food & Drug Administration (FDA). (2023). Drug Approval Processes.
- European Medicines Agency (EMA). (2023). Regulatory Procedures for Pharmaceuticals.
- Business Monitor International (BMI). (2022). Pharmaceutical Market Outlook.
- Pharma Intelligence. (2023). R&D Trends in Respiratory Therapeutics.
More… ↓
