Share This Page
Drug Price Trends for CAVERJECT IMPULSE
✉ Email this page to a colleague
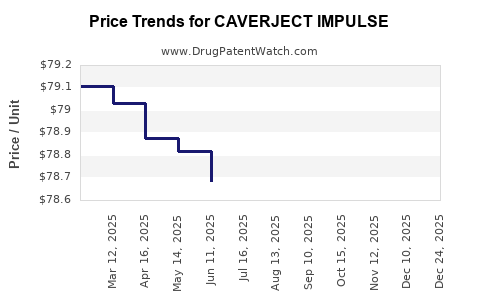
Average Pharmacy Cost for CAVERJECT IMPULSE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| CAVERJECT IMPULSE 10 MCG KIT | 00009-5181-01 | 78.68545 | EACH | 2025-11-19 |
| CAVERJECT IMPULSE 20 MCG KIT | 00009-5182-01 | 101.33136 | EACH | 2025-11-19 |
| CAVERJECT IMPULSE 10 MCG KIT | 00009-5181-01 | 78.68773 | EACH | 2025-10-22 |
| CAVERJECT IMPULSE 20 MCG KIT | 00009-5182-01 | 101.33136 | EACH | 2025-10-22 |
| CAVERJECT IMPULSE 20 MCG KIT | 00009-5182-01 | 101.00889 | EACH | 2025-09-17 |
| CAVERJECT IMPULSE 10 MCG KIT | 00009-5181-01 | 78.56409 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for CAVERJECT IMPULSE
Introduction
CAVERJECT IMPULSE (alprostadil injection) stands as a critical therapeutic option for erectile dysfunction (ED), primarily marketed via the pioneering delivery system that enhances patient convenience and compliance. As a rapidly evolving segment within urology and men's health, understanding CAVERJECT IMPULSE's current market landscape, competitive positioning, regulatory environment, and future pricing trajectory is vital for healthcare stakeholders, investors, and policymakers.
Market Overview
Therapeutic Profile and Indication
CAVERJECT IMPULSE contains alprostadil, a prostaglandin E1 derivative, approved by the FDA for intracavernosal injection therapy for ED in adult men. The drug is distinguished by its direct mechanism—vasodilation of penile arteries ensuring increased blood flow—offering an effective alternative for patients unresponsive to oral phosphodiesterase type 5 inhibitors (PDE5 inhibitors). Its rapid onset and high efficacy underpin its central role in treatment-resistant cases, especially where oral medications are contraindicated.
Market Size and Drivers
The global ED market was valued at approximately USD 4.3 billion in 2022, with expectations to reach USD 7.0 billion by 2030, at a CAGR of about 6.0% [1]. CAVERJECT IMPULSE holds a niche, yet significant, segment within this broader market, particularly among patients requiring injectable therapy. Key market drivers include rising aging populations, increased awareness of ED treatment options, and the expanding acceptance of minimally invasive therapies.
Competitive Landscape
CAVERJECT IMPULSE faces competition from other injectable formulations such as EDex (injectable alprostadil), compounded injections, and emerging non-invasive solutions like low-intensity shockwave therapy and newer oral agents with improved efficacy. Its main differentiators include its proprietary delivery device—cPercShow, which simplifies administration—and its established safety profile.
Regulatory and Reimbursement Environment
The drug benefits from widespread FDA approval and a robust reimbursement framework across developed markets, including Medicare and private insurance, facilitating its market penetration. However, reimbursement policies can vary geographically, influencing price and market access.
Market Dynamics and Challenges
Patient Preferences and Compliance
Injectable therapy may deter some patients due to invasiveness and discomfort, impacting overall market share. CAVERJECT IMPULSE’s innovative delivery device aims to mitigate these barriers, promoting better adherence.
Pricing Sensitivity and Cost Considerations
Cost remains a key factor influencing prescribing patterns—both for payers and patients. Premium pricing models are common for injectables, justified by their high efficacy and convenience factors. However, competitive pricing becomes pivotal as oral alternatives become more effective and accessible.
Market Penetration and Adoption
Physician familiarity and patient education impact adoption rates. With ongoing educational efforts, acceptance is steadily improving, especially in cases with refractory ED.
Price Projections and Future Trends
Current Pricing Landscape
As of 2023, the average wholesale price (AWP) of CAVERJECT IMPULSE ranges from USD 150 to USD 250 per injection, depending on dosage and geographic region [2]. Insurance reimbursement often reduces patient out-of-pocket expenses, but disparities exist.
Projected Price Trends (2023-2030)
- Moderate Price Stability: Given its status as a branded, patent-protected product, significant price reductions are unlikely before patent expiry or biosimilar entry. However, generic competition is minimal at present.
- Potential Premium Pricing: Continued innovation in delivery devices and formulation enhancements could justify premium pricing strategies to sustain revenue growth.
- Impact of Biosimilars and Generics: Patent expirations or regulatory pathways permitting biosimilar development could introduce lower-cost alternatives, pressuring prices downward post-2030.
Market Expansion and Pricing Impacts
The expanding global market, especially in emerging economies like China and India, where ED prevalence is rising and healthcare infrastructure improves, may sustain higher prices initially due to limited competition. Conversely, price erosion may occur as generics or biosimilars enter these markets.
Influencing Factors on Future Pricing
- Regulatory developments: Approval of biosimilars or alternative delivery systems.
- Healthcare policy shifts: Price caps and value-based reimbursement models.
- Innovation: New formulations or delivery devices enhancing convenience.
- Market penetration: Increased patient and physician acceptance.
Strategic Considerations for Stakeholders
- Manufacturers: Focus on technological innovations and targeted marketing to justify premium pricing.
- Payers: Assess value-based models, considering efficacy and quality-of-life improvements against costs.
- Patients: Educational initiatives can expand acceptance, influencing demand and pricing elasticity.
Key Takeaways
- CAVERJECT IMPULSE occupies a vital niche within the growing ED therapeutics market, leveraging its innovative delivery system and proven efficacy.
- Its current price trajectory remains relatively stable due to patent protections and brand positioning but faces future pressures from potential biosimilar entrants.
- The expanding global market, demographic shifts, and ongoing innovation present opportunities for strategic pricing but necessitate careful navigation of competitive and regulatory landscapes.
- Healthcare providers and payers must balance cost considerations with clinical benefits, increasingly adopting value-based reimbursement models.
- Long-term pricing stability hinges on technological innovation, market expansion, and evolving regulatory policies.
FAQs
1. What distinguishes CAVERJECT IMPULSE from other ED treatments?
Its novel delivery device simplifies administration of alprostadil, reducing injection pain and improving patient adherence compared to traditional methods.
2. How might upcoming biosimilar products impact CAVERJECT IMPULSE pricing?
Introduction of biosimilars can lead to significant price reductions post-patent expiry, increasing market competition and potentially lowering costs.
3. What geographic regions present the most significant growth opportunities for CAVERJECT IMPULSE?
Emerging economies like China and India, driven by rising ED prevalence and improving healthcare access, represent substantial growth prospects with sustained premium pricing initially.
4. How do insurance reimbursement policies influence the pricing of injectables like CAVERJECT IMPULSE?
Reimbursement frameworks often determine patient out-of-pocket costs, impacting demand. Favorable reimbursement can support stable or premium pricing, while restrictions can pressure prices downward.
5. What future innovations could affect CAVERJECT IMPULSE's market positioning?
Advances in non-invasive ED therapies, improved delivery systems, or combination treatments could diminish demand for injectable options, influencing long-term pricing strategies.
References
[1] MarketResearch.com, "Global Erectile Dysfunction Drugs Market,” 2022.
[2] GoodRx, "Caverject (alprostadil) injectable prices," 2023.
More… ↓
