Share This Page
Drug Price Trends for BACTERIOSTATIC WATER VIAL
✉ Email this page to a colleague
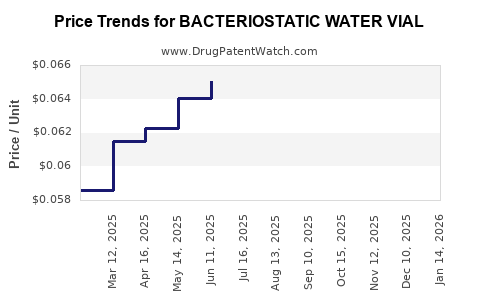
Average Pharmacy Cost for BACTERIOSTATIC WATER VIAL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| BACTERIOSTATIC WATER VIAL | 00409-3977-03 | 0.08601 | ML | 2025-12-17 |
| BACTERIOSTATIC WATER VIAL | 00409-3977-03 | 0.08291 | ML | 2025-11-19 |
| BACTERIOSTATIC WATER VIAL | 00409-3977-03 | 0.08220 | ML | 2025-10-22 |
| BACTERIOSTATIC WATER VIAL | 00409-3977-03 | 0.07846 | ML | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Bacteriostatic Water Vial
Introduction
Bacteriostatic water vials are sterile, non-preserved water solutions used primarily for diluting or dissolving medications administered via injection. Widely utilized in hospitals, clinics, pharmacies, and research laboratories, these vials are critical for ensuring patient safety in injectable drug preparation. As the pharmaceutical and healthcare sectors evolve, understanding the product's market landscape and future pricing trends becomes imperative for pharmaceutical companies, healthcare providers, and investors. This analysis delves into the current market dynamics, competitive landscape, and price projections for bacteriostatic water vials over the next five years.
Market Overview
Demand Drivers
The demand for bacteriostatic water vials largely correlates with the growth in injectable drug therapies, particularly in the realms of endocrinology, oncology, and anesthesiology. The increasing prevalence of chronic diseases necessitating injectable medications and the proliferation of compounded medications sustain ongoing demand.
In 2022, the global injectable drug market was valued at approximately $600 billion, with a compound annual growth rate (CAGR) of around 7% predicted until 2027 [1]. Bacteriostatic water is indispensable in this segment for safe medication reconstitution, thereby directly aligning its demand with the broader injectable market.
Regional Market Dynamics
- North America: The dominant market, driven by stringent regulatory standards, high healthcare expenditure, and a large number of compounding pharmacies.
- Europe: Similar growth patterns with expanding hospital infrastructure and rising awareness regarding sterile practices.
- Asia-Pacific: The fastest-growing region, propelled by increasing healthcare infrastructure, rising incidences of chronic diseases, and expanding pharmaceutical manufacturing capacity.
Market Segmentation
Based on type, bottle size, and end-user:
- Type: 30 mL, 100 mL, and 240 mL vials.
- End-user: Hospitals, clinics, research laboratories, compounding pharmacies.
- Distribution channels: Direct sales, online pharmacies, medical wholesalers.
Competitive Landscape
The market comprises several key players, including:
- Baxter International Inc.
- B. Braun Melsungen AG
- Fresenius Kabi AG
- Respectively, these companies focus on sterile pharmaceutical products and have significant manufacturing and distribution capabilities worldwide [2].
Generic manufacturers and regional suppliers also contribute to market diversity, often offering competitively priced bacteriostatic water vials.
Regulatory and Quality Standards
Compliance with regulatory standards such as the FDA (U.S.), EMA (Europe), and pharmacopoeial standards (USP, EP) influences market entry and pricing. The emphasis on sterile manufacturing practices ensures safety but also affects costs and, subsequently, prices.
Market Challenges
- High manufacturing costs due to strict aseptic processing.
- Regulatory hurdles across different regions.
- Competition from alternative diluents or IV solutions, albeit limited.
Price Analysis and Trends
Current Pricing Landscape
Pricing of bacteriostatic water vials varies significantly depending on geographic location, vial size, brand reputation, and procurement channels.
- In the U.S., a 30 mL bacteriostatic water vial typically retails at approximately $2.50 to $4.00 per unit.
- Larger sizes, such as 100 mL vials, may range between $4.00 to $8.00.
- Regional disparities, especially in emerging markets, tend to feature lower prices driven by local manufacturing and lower regulatory costs.
Factors Influencing Pricing
- Manufacturing costs: Compliance with sterile manufacturing increases production expenses, which are reflected in pricing.
- Brand versus generic: Established branded products often command premium pricing, while generics compete aggressively on price.
- Regulatory approval and quality standards: Stringent regulatory compliance increases product costs but assures safety, influencing consumer willingness to pay.
- Supply chain dynamics: Disruptions, such as raw material shortages or logistical constraints, can impact pricing stability.
Projected Price Trends (2023–2028)
Based on current market trajectories:
- Moderate Price Stability: The base case indicates minimal fluctuations in unit prices, barring significant supply chain disruptions or regulatory changes.
- Potential Price Increases: Due to inflationary pressures and rising manufacturing costs, an average annual increase of approximately 2-3% is plausible.
- Market Diversification Effect: Entry of low-cost regional manufacturers may exert downward pressure, fostering price competition.
Future Market Outlook
Growth Projections
- The global market size for sterile water and diluents is expected to reach USD 1.2 billion by 2028, growing at a CAGR of around 4.5% [3].
- Bacteriostatic water vials will account for a significant proportion of this segment, driven by increasing injectable therapies.
- The expansion of outpatient clinics and home-care injectable solutions could further augment demand.
Technological and Regulatory Innovations
- Adoption of single-use, environmentally sustainable packaging might influence manufacturing costs and, consequently, prices.
- Streamlined regulatory procedures and international harmonization could reduce compliance costs, potentially leading to more competitive pricing.
Market Entry and Pricing Strategy Recommendations
- For new entrants, emphasizing cost-effective manufacturing and compliance with international standards will be essential.
- Embracing digital distribution channels and establishing partnerships with regional suppliers can maintain competitive pricing.
- High-volume procurement by institutional clients warrants differentiated pricing models or bulk discounts.
Key Takeaways
- The bacteriostatic water vial market is anchored to the broader growth of injectable pharmaceuticals.
- Prices vary geographically, with North America commanding premium pricing due to regulatory and quality standards.
- Market dynamics favor moderate price increases driven by inflation and manufacturing costs.
- Increased competition from regional generic suppliers may exert downward pricing pressure.
- Innovations in packaging and streamlined regulatory processes offer opportunities to optimize pricing and margins.
Conclusion
The future of the bacteriostatic water vial market appears stable with steady growth linked to the expanding injectable drug sector. Price projections indicate modest increases, influenced by manufacturing and regulatory factors, while geopolitical and supply chain considerations may introduce variability. Stakeholders should adopt flexible, compliance-oriented strategies to capitalize on market opportunities and mitigate risks.
FAQs
1. What factors most influence the pricing of bacteriostatic water vials?
Manufacturing costs, regulatory compliance, brand reputation, vial size, and regional market conditions significantly impact pricing.
2. How does regional regulation affect the market price?
Stringent regulatory standards entail higher manufacturing expenses, leading to elevated prices in regions like North America and Europe compared to emerging markets.
3. Are there alternative products to bacteriostatic water vials?
While alternatives such as sterile saline solutions exist, bacteriostatic water's specific preservative properties make it uniquely suited for certain injectable preparations.
4. What is the growth outlook for this market?
The market is projected to grow at a CAGR of 4.5% until 2028, driven by increased injectable therapies and healthcare infrastructure expansion.
5. How might technological innovations influence future prices?
Technological improvements in manufacturing and packaging could reduce costs, fostering competitive pricing and improved product safety.
References
[1] MarketWatch. "Global Injectable Drug Market Size, Share & Trends Analysis Report 2022–2027."
[2] PharmaMarketReport. "Leading Manufacturers in Sterile Pharmaceutical Products."
[3] Grand View Research. "Sterile Water and Diluent Market Size, Share & Trends Analysis."
More… ↓
