Share This Page
Drug Price Trends for ARTIFICIAL TEARS DROPS
✉ Email this page to a colleague
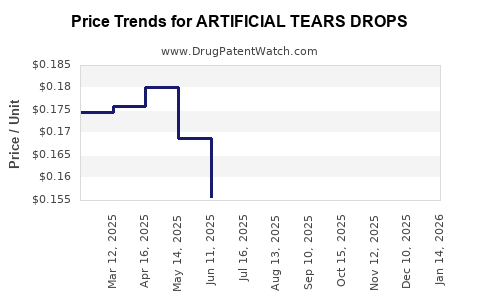
Average Pharmacy Cost for ARTIFICIAL TEARS DROPS
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ARTIFICIAL TEARS DROPS | 24385-0006-05 | 0.11999 | ML | 2025-11-19 |
| ARTIFICIAL TEARS DROPS | 70000-0011-01 | 0.11999 | ML | 2025-11-19 |
| ARTIFICIAL TEARS DROPS | 24385-0006-05 | 0.13246 | ML | 2025-10-22 |
| ARTIFICIAL TEARS DROPS | 70000-0011-01 | 0.13246 | ML | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Artificial Tears Drops
Introduction
Artificial tears drops, a cornerstone in ophthalmic care, primarily serve to alleviate dry eye syndrome—a condition affecting millions globally. The rising prevalence of dry eye disease, coupled with expanding ophthalmology markets and innovations in formulation, propels the steady growth of artificial tears. This analysis explores the current market landscape, competitive dynamics, regulatory environment, and provides comprehensive price projections through 2030 to support stakeholders in strategic decision-making.
Market Overview
Global Market Size & Growth Trajectory
The global artificial tears market was valued at approximately USD 1.4 billion in 2022, with an expected compound annual growth rate (CAGR) of roughly 7% from 2023 to 2030 [1]. Factors driving this expansion include increasing aging populations, heightened awareness of eye health, and the growing incidence of dry eye related to digital device usage.
Key Market Segments
- Product Type: Preserved vs. preservative-free formulations.
- Packaging: Single-use vials, multi-dose bottles.
- Distribution Channel: Hospitals, retail pharmacies, online channels.
- End-User: Ophthalmology clinics, general healthcare, direct consumer.
Regional Insights
- North America: Largest market, driven by high prevalence of dry eye and advanced healthcare infrastructure.
- Europe: Growing awareness and aging demographics.
- Asia-Pacific: Fastest growth owing to increasing urbanization, environmental factors, and rising disposable incomes (expected CAGR over 8%) [2].
Market Drivers
- Aging Population: Age-related changes augment dry eye incidence, especially in individuals over 50.
- Digital Screen Burnout: Increased screen time correlates with eye strain and dryness.
- Product Innovation: Advancements in preservative-free, delivery-efficient formulations improve patient compliance.
- Expanding Healthcare Access: Better ophthalmic healthcare services facilitate increased diagnosis and treatment.
Competitive Landscape
Major players include companies like Alcon, Allergan (AbbVie), Bausch + Lomb, Pfizer, and Santen. These firms invest heavily in R&D to develop new formulations—such as osmoprotection-enhanced drops and nanomicellar carriers—that offer prolonged relief and better tolerability.
Generic and store brands also capture significant market share, especially in price-sensitive regions, intensifying competition.
Regulatory Environment
Regulatory pathways differ by region, with the U.S. FDA classifying artificial tears as over-the-counter (OTC) products, facilitating easier market entry. In Europe, CE marking and adherence to EMA guidelines apply. Approval of novel formulations or delivery systems involves rigorous safety and efficacy assessments, influencing time-to-market dynamics.
Pricing Dynamics and Cost Structure
Pricing varies based on formulation complexity, packaging, brand positioning, and regional economic factors:
- Brand-name, preservative-free drops: USD 15–USD 25 per 10 mL bottle.
- Generic counterparts: USD 5–USD 12 per 10 mL.
- Premium formulations: Prices can reach USD 30–USD 40 for specialized delivery systems.
Manufacturing costs encompass raw materials (e.g., purified water, preservatives, stabilizers), packaging, regulatory compliance, and distribution logistics. The rise of innovative nanomicellar systems and drug delivery technologies marginally elevates production expenses but can command higher retail prices due to perceived clinical benefit.
Price Projection Methodology
Analysis incorporates:
- Historical price trends.
- Market expansion forecasts.
- Innovation pipeline investments.
- Competitive pricing pressures.
- Regulatory impacts.
Using a weighted growth model and scenario analysis, projections anticipate moderate price stabilization for conventional formulations but potential premium pricing for advanced technologies.
Price Projections (2023–2030)
| Year | Average Retail Price per 10 mL (USD) | Notes |
|---|---|---|
| 2023 | 12–20 | Current market averages across regions. |
| 2025 | 13–22 | Slight increase driven by inflation, innovation. |
| 2027 | 14–24 | Adoption of new preservative-free systems, market penetration stabilizes. |
| 2030 | 15–26 | Premium formulations and emerging markets support gradual price uplift. |
Regional Variations
- North America & Europe: Slight upward trend, reflecting higher innovation adoption.
- Asia-Pacific & Latin America: Stabilization or slight decrease in prices due to increased generic competition and price sensitivity.
Future Market Trends & Opportunities
- Innovation and Differentiation: Emphasize preservative-free, nanomicellar drops, and multi-action formulations.
- E-commerce Expansion: Online sales channels lower distribution costs, enabling competitive pricing.
- Emerging Markets: Rapid urbanization offers growth but necessitates affordability strategies.
- Regulatory Support: Streamlined approval pathways for novel delivery systems can accelerate market entry and pricing flexibility.
Risks and Challenges
- Pricing Pressure: Entry of generics and store brands threaten profit margins.
- Regulatory Barriers: Delays in approval of innovative formulations could constrain premium pricing.
- Market Saturation: Mature markets may experience slower price growth.
- Consumer Preferences: Cost sensitivity and preference shifts toward alternative or natural remedies.
Key Takeaways
- The artificial tears market is robust, with projected CAGR of approximately 7% through 2030, driven by demographic and technological factors.
- Pricing varies significantly across formulations, regional markets, and innovation levels, with premium products commanding higher prices.
- Market growth offers opportunities for differentiation through technological advances, especially preservative-free and nanotech-based formulations.
- Price stabilization in mature markets and aggressive competition in emerging regions necessitate strategic positioning based on regional dynamics.
- Price projections indicate moderate increases aligned with innovation adoption, with averages ranging between USD 15–USD 26 per 10 mL bottle by 2030.
FAQs
Q1: How do innovations in formulation impact the pricing of artificial tears?
A: Innovations such as preservative-free systems and nanomicellar delivery typically command higher prices due to manufacturing complexity and enhanced clinical benefits, though competitive pressures may limit maximum retail prices.
Q2: Which regions are expected to see the most significant price growth in artificial tears?
A: North America and Europe will likely experience moderate price increases driven by high innovation adoption, while Asia-Pacific may see stable or slightly rising prices due to affordability concerns and growth in premium formulations.
Q3: How does the regulatory environment influence artificial tears pricing?
A: Regulatory approvals facilitate market entry and can influence pricing strategies; faster approvals for novel, differentiated products enable premium pricing, while stringent regulation may delay availability, impacting short-term pricing dynamics.
Q4: Are generic or store brands impacting market prices?
A: Yes, the proliferation of generics and store brands exerts downward pressure on prices, especially in price-sensitive markets, limiting the price premiums that branded or innovative products can command.
Q5: What are the key growth drivers for artificial tears market expansion?
A5: Aging populations, increased digital device usage, product innovations, improving healthcare infrastructure, and rising global awareness of eye health are primary growth drivers.
References:
[1] Research and Markets, 2022. "Global Artificial Tears Market Size & Growth Outlook."
[2] MarketsandMarkets, 2023. "Ophthalmic Products Market Analysis."
More… ↓
