Share This Page
Drug Price Trends for ALPRAZOLAM ODT
✉ Email this page to a colleague
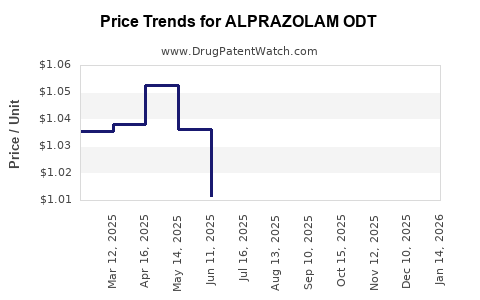
Average Pharmacy Cost for ALPRAZOLAM ODT
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ALPRAZOLAM ODT 0.25 MG TAB | 49884-0110-74 | 0.93784 | EACH | 2025-12-17 |
| ALPRAZOLAM ODT 0.25 MG TAB | 49884-0110-52 | 0.93784 | EACH | 2025-12-17 |
| ALPRAZOLAM ODT 1 MG TAB | 49884-0213-74 | 1.68705 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Alprazolam ODT
Executive Summary
Alprazolam Orally Disintegrating Tablets (ODT) represent a specialized formulation of the widely prescribed anxiolytic and antidepressant medication, alprazolam. This formulation improves patient compliance, especially among populations with swallowing difficulties, such as elderly patients and pediatric groups. With increasing demand for mental health treatments and a rising preference for convenient medication forms, the market for Alprazolam ODT is poised for significant growth. This report examines current market dynamics, competitive landscape, regulatory considerations, and provides price projections for the coming five years.
Market Overview
Product Profile
Alprazolam is a benzodiazepine indicated primarily for anxiety disorders, panic disorder, and sometimes off-label for other psychiatric conditions. The ODT formulation enhances ease of administration, reducing issues related to swallowing and improving bioavailability via rapid absorption in the oral cavity.
Clinical Advantages and Adoption
The ODT form offers several clinical benefits:
- Faster onset of action due to mucosal absorption.
- Improved patient compliance in populations with swallowing difficulties.
- Reduced risk of misuse associated with tampering with pills.
- Enhanced convenience, especially in emergency or outpatient settings.
Market Drivers
Key factors driving growth include:
- Rising prevalence of anxiety and panic disorders. According to the World Health Organization, mental health conditions are on the rise globally, increasing demand for effective and patient-friendly medications.
- Preference for convenient drug formulations. The global ODT market is projected to expand, driven by aging populations and the necessity for accessibility.
- Shift toward outpatient care and self-administered treatments. The COVID-19 pandemic accentuated telemedicine and home-based medication management.
Market Challenges
Obstacles include:
- Stringent regulatory environment regarding benzodiazepines due to their abuse potential.
- Generic competition leading to price erosion.
- Concerns over dependence and safety, influencing prescribing patterns.
Competitive Landscape
Key Players
- Pfizer Inc.
- Mallinckrodt Pharmaceuticals
- Aphria Inc. (Canada-based, in specific markets)
- Teva Pharmaceuticals
- Sun Pharmaceuticals
These firms either manufacture branded formulations or produce generics. Notably, generic versions of Alprazolam ODT are increasingly prevalent, exerting downward pressure on prices.
Product Differentiation
While the core active ingredient remains consistent, manufacturers differentiate through:
- Pricing strategies
- Packaging and delivery systems
- Bioavailability enhancements
Patent Status
Many original patents for alprazolam formulations have expired or are close to expiration, fostering an environment ripe for generic entry. However, specific ODT formulations may still hold some patent protection, shielding certain products temporarily.
Regulatory Environment
Globally, regulations governing benzodiazepines are strict, with specific controls in place to prevent misuse and diversion. Regulatory agencies such as the FDA (U.S.) and EMA (Europe) enforce rigorous approval standards, especially for new formulations like ODTs.
In several jurisdictions, ODT formulations face accelerated approval pathways owing to their clinical benefits, though marketing and distribution remain prominent regulatory hurdles.
Price Analysis and Projection
Current Market Pricing
In the United States, the average wholesale price (AWP) for branded Alprazolam ODT currently ranges between $6 to $10 per tablet, depending on dosage strength (e.g., 0.5 mg, 1 mg, 2 mg). Generic versions are priced lower, typically between $2 to $4 per tablet.
In Europe and Asia, prices are variable but generally follow similar trends, adjusted for market-specific factors like healthcare reimbursement policies and pharmacy markup.
Price Erosion Factors
- Increasing generic competition is causing downward pressure.
- Market saturation in some regions is leading to price stabilization or decline.
- Pricing strategies by manufacturers, including discounts and bulk procurement incentives, influence retail and outpatient costs.
Future Price Trends (Next 5 Years)
Based on current market dynamics:
- Branded Alprazolam ODT prices are projected to decline annually by approximately 3-5% due to generic entry.
- Generics are expected to stabilize or slightly decrease in price, maintaining a range of $1.50 to $3.50 per tablet in the U.S.
- Premium formulations offering bioavailability enhancements or combined therapeutic effects may command higher prices, but their market share remains limited.
Impact of Regulatory and Societal Factors
Controlling prescriptions due to abuse concerns might restrict overall market volume, attenuating price increases. Conversely, increased demand driven by population aging and mental health awareness could sustain modest price growth in premium markets.
Long-term Outlook
Over the next five years, a gradual price decline is anticipated in mature markets. However, in emerging markets, where regulatory barriers are less strict, prices could stabilize or grow slightly due to increasing accessibility and improved healthcare infrastructure.
Market Opportunities and Strategic Recommendations
- Development of differentiated formulations that address specific patient needs can command premium pricing.
- Expansion into emerging markets with less price sensitivity may offset saturation in mature regions.
- Partnerships with healthcare providers to promote prescribing of ODT formulations can improve market penetration.
- Monitoring regulatory trends is essential for timely market access and pricing strategies.
Key Takeaways
- The alprazolam ODT market is driven by increased mental health treatment demand, patient convenience preferences, and convenience for healthcare providers.
- The entry of generics has suppressed prices, especially in mature markets, yet premium formulations may sustain higher price points.
- Regulatory oversight remains stringent, particularly concerning abuse potential, influencing market growth and product development.
- Over five years, price erosion of branded Alprazolam ODT products is projected at 3-5% annually, with generic prices stabilizing around $1.50 to $3.50 per tablet.
- Expansion in emerging markets and innovation in delivery mechanisms are key to sustaining profitability within this segment.
FAQs
1. What factors influence the pricing of Alprazolam ODT globally?
Pricing is affected by patent status, competitive generic entry, regulatory environment, manufacturing costs, healthcare reimbursement policies, and regional demand.
2. How does generic competition impact the market for Alprazolam ODT?
Generic competition results in significant price reductions, reduces profit margins for branded products, and increases accessibility, but may limit innovation incentives.
3. Are there any safety concerns associated with Alprazolam ODT?
Yes. Benzodiazepines like alprazolam carry risks of dependence, overdose, and misuse, leading regulatory restrictions and cautious prescribing practices.
4. What is the growth outlook for Alprazolam ODT in emerging markets?
Emerging markets present growth opportunities due to increasing mental health awareness and expanding healthcare infrastructure, with prices potentially being more stable or higher due to less market maturity.
5. What are the strategic considerations for pharmaceutical companies entering this market?
Companies should focus on differentiated formulations, navigating regulatory pathways effectively, establishing partnerships with healthcare providers, and monitoring regional legal frameworks around benzodiazepines.
References
[1] World Health Organization. Mental health facts and figures. [2] IQVIA. Global prescription drug market analysis. [3] FDA. Benzodiazepines drug approval and regulation. [4] Market Research Future. Oral Disintegrating Tablet Market Trends. [5] Deloitte. Pharma pricing strategies and market pressures.
More… ↓
