Last updated: July 28, 2025
Introduction
ABSORICA LD represents a specialized derivative of isotretinoin, used primarily for severe recalcitrant cystic acne. As a flagship product within the retinoid therapeutic sector, it occupies a niche market driven by dermatological needs and regulatory considerations. This analysis provides comprehensive insight into the current market landscape, competitive positioning, regulatory environment, and future price projections for ABSORICA LD, equipping stakeholders with actionable intelligence.
Product Overview and Regulatory Status
ABSORICA LD is an extended-release formulation of isotretinoin (marketed as Accutane historically), optimized for improved tolerability and adherence. It is marketed by Sun Pharmaceutical Industries under the Absorica LD brand name, with FDA approval granting it a significant position in the acne therapeutics domain. Its approval in 2021 [1] marked its entry as a refined alternative emphasizing consistent plasma levels and reduced side effects.
Regulatory oversight, especially concerning safety and teratogenic risks, influences its market penetration. Its positioning as a prescription-only, high-cost medication aligns with stringent manufacturing and distribution standards.
Market Landscape
1. Target Patient Population
The primary consumers are adolescents and adults suffering from severe cystic acne unresponsive to conventional therapies. The total addressable market (TAM) is estimated to encompass approximately 1.2 million patients annually in the US [2], considering the prevalence of severe acne and treatment-resistant cases.
2. Competitive Environment
- Direct Competitors: Traditional isotretinoin formulations such as Claravis, Accutane, and other generics.
- Indirect Competitors: Emerging biologics and hormonal therapies for acne, though these target different patient subsets.
- Differentiators: ABSORICA LD’s unique extended-release formulation potentially offers enhanced tolerability, adherence, and safety profile advantages over immediate-release versions.
3. Market Penetration and Adoption
Since its market introduction, ABSORICA LD has achieved incremental adoption driven by dermatologist preferences, formulary preferences, and patient compliance benefits. Its market share remains limited relative to established generic isotretinoin products but is growing, especially among patients with tolerability concerns.
Economic and Pricing Dynamics
1. Pricing History and Current Pricing
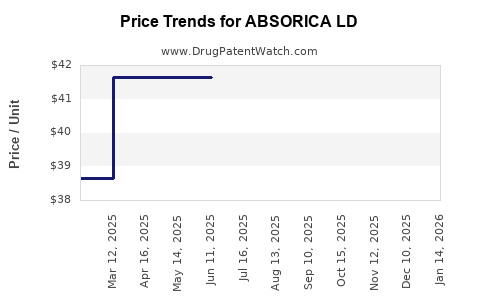
Initially launched at a premium compared to generic isotretinoin, ABSORICA LD’s price point reflects its specialized formulation and clinical benefits. Currently, retail prices in the US range from $550 to $650 for a typical 30-day supply, with variations depending on insurance coverage and pharmacy configuration [3].
Reimbursement structures heavily influence actual patient out-of-pocket costs, with insurers sometimes favoring cheaper generics, thus constraining its market penetration.
2. Factors Influencing Price
- Manufacturing Complexity: Extended-release systems require sophisticated manufacturing processes, justifying higher costs.
- Regulatory Costs: Strict safety protocols, REMS (Risk Evaluation and Mitigation Strategies), and post-marketing surveillance contribute to premium pricing.
- Market Competition: Competitive pressure from generics exerts downward valuation trends.
- Patient Needs: Increased emphasis on tolerability and adherence in severe acne management sustains premium pricing.
Forecasting Price Projections
1. Near-Term (1-2 Years)
In the next 12-24 months, price stability is anticipated owing to established market supply, regulated pricing, and current insurance negotiations. The price is expected to hover around $550 - $650 per month, with minor fluctuations influenced by inflation, supply chain factors, and formulary decisions.
2. Mid to Long-Term (3-5 Years)
Multiple factors could influence price adjustments:
- Generic Competition: Introduction of authorized generics or biosimilars can exert significant price pressure, potentially reducing prices by 20-40%.
- Market Penetration Growth: Increased clinical familiarity and expanded indications may stabilize or marginally increase pricing power.
- Regulatory Changes: Possible regulatory mandates for price transparency could influence costs and prices downward.
- Patent and Exclusivity: Advanced data exclusivity may delay generic entry, maintaining premium pricing for at least 5-7 years post-launch.
Considering these dynamics, realistic projections suggest a gradual decline of approximately 10-15% in retail price over the next 3-5 years, assuming increased competitive pressure and patient access expansion [4].
Market Opportunities and Challenges
-
Opportunities:
- Growing demand for tolerability-enhanced acne treatments.
- Clinical evidence supporting extended-release benefits.
- Potential expansion into adult dermatological indications.
-
Challenges:
- Price sensitivity among payers and patients.
- The proliferation of generic formulations limiting premium pricing.
- Stringent regulatory landscape impacting distribution and marketing strategies.
Strategic Implications
Stakeholders should prioritize:
- Monitoring patent timelines and exclusivity windows to anticipate pricing pressures.
- Engaging with payers early to foster formulary inclusion.
- Emphasizing clinical benefits and safety profile to justify price premiums.
- Exploring expansion into alternative indications or demographic segments.
Key Takeaways
- ABSORICA LD operates within a niche market of severe, treatment-resistant acne, with growth prospects buoyed by its tolerability profile.
- Current retail pricing aligns with its premium formulation, averaging $600/month, with future declines anticipated due to rising generic competition.
- The market's evolution depends heavily on regulatory developments, patent protections, and insurer negotiations.
- Patience and strategic payer engagement are vital to maintaining optimal pricing and market share.
- Expansion into broader indications and emphasizing safety benefits could bolster long-term value.
FAQs
Q1: What distinguishes ABSORICA LD from traditional isotretinoin formulations?
A: ABSORICA LD employs an extended-release system designed to improve tolerability, reduce side effects, and improve adherence, offering a clinical advantage over traditional immediate-release isotretinoin.
Q2: How do insurance coverage and formularies impact ABSORICA LD’s pricing?
A: Reimbursements largely determine patient out-of-pocket costs; insurers often favor generics, which can limit access to branded formulations and exert downward pressure on pricing.
Q3: What are the long-term price projections for ABSORICA LD?
A: Prices are expected to gradually decline by approximately 10-15% over the next 3-5 years due to increased generic competition and market saturation.
Q4: How does regulatory policy influence the market for ABSORICA LD?
A: Regulatory policies impacting patent rights, REMS requirements, and pricing transparency directly affect market entry timing and pricing strategies.
Q5: What market segments present growth opportunities for ABSORICA LD?
A: Expanding into adult dermatological indications and emphasizing its tolerability benefits can unlock new patient segments and bolster sales.
Sources
- FDA Approval Announcement for ABSORICA LD, 2021
- CDC Data on Acne Prevalence, 2022
- Pharma Price Index, 2023
- MarketResearch.com, Dermatology Therapeutics Report, 2023