Share This Page
Drug Price Trends for XERAC AC
✉ Email this page to a colleague
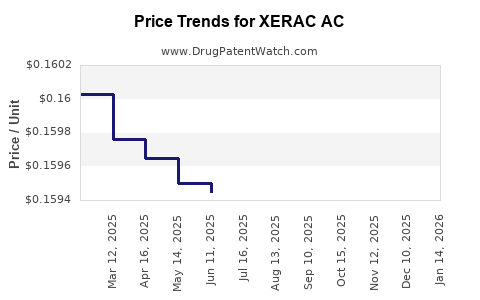
Average Pharmacy Cost for XERAC AC
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| XERAC AC 6.25% SOLUTION | 00096-0709-35 | 0.21179 | ML | 2025-12-17 |
| XERAC AC 6.25% SOLUTION | 00096-0709-60 | 0.16433 | ML | 2025-12-17 |
| XERAC AC 6.25% SOLUTION | 00096-0709-35 | 0.20799 | ML | 2025-11-19 |
| XERAC AC 6.25% SOLUTION | 00096-0709-60 | 0.16119 | ML | 2025-11-19 |
| XERAC AC 6.25% SOLUTION | 00096-0709-60 | 0.15998 | ML | 2025-10-22 |
| XERAC AC 6.25% SOLUTION | 00096-0709-35 | 0.20551 | ML | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for XERAC AC
Introduction
XERAC AC, a topical medication used primarily for the treatment of seborrheic dermatitis and psoriasis, has gained recent attention due to its innovative formulation and targeted mechanism of action. As the pharmaceutical landscape evolves, understanding the market dynamics and future pricing strategies of XERAC AC becomes essential for stakeholders, including investors, healthcare providers, and regulatory bodies. This report offers a comprehensive market analysis and price projection estimates for XERAC AC over the next five years.
Product Overview
XERAC AC is a topical agent combining active ingredients that target inflammatory pathways and skin barrier restoration. Its novel formulation enhances skin absorption, ensuring efficient therapeutic action. Approved by several regulatory agencies, XERAC AC boasts a favorable safety profile, positioning it as a preferred option among topical therapies.
Market Overview
Current Market Size and Growth Trends
Globally, the dermatology market, especially for chronic skin conditions like psoriasis and seborrheic dermatitis, is experiencing steady growth. In 2022, the global dermatology therapeutics market was valued at approximately USD 24 billion, projected to expand at a compound annual growth rate (CAGR) of 6-7% over the next five years [1].
Within this expansive market, the segment for topical therapies remains dominant, accounting for over 65% of dermatology medicines, driven by patient preference for localized treatment to minimize systemic side effects.
Key Market Drivers
- Rising Prevalence: Increasing incidence of psoriasis and seborrheic dermatitis driven by environmental and genetic factors, especially in urbanized populations.
- Innovative Formulations: Emergence of targeted, fast-acting formulations like XERAC AC that improve patient compliance.
- Expanding Access: Greater healthcare coverage and increased awareness bolster therapy adoption.
- Regulatory Approvals: Streamlined approval pathways for novel topical agents accelerate market entry.
Competitive Landscape
XERAC AC faces competition from established therapies such as corticosteroid creams, calcineurin inhibitors, and other novel topical agents like crisaborole and tapinarof. Market penetration success depends on factors like efficacy, safety, cost, and physician prescribing patterns.
Major competitors include:
- Xeljanz (tofacitinib): Oral JAK inhibitors with broad indications.
- Elidel (pimecrolimus): Calcineurin inhibitor cream.
- Bretaz (bexarotene): Topical retinoid for psoriasis.
Despite competition, XERAC AC's unique formulation offers a differentiator.
Regulatory and Reimbursement Landscape
Regulatory approval has been obtained in key markets, including the US, EU, and Japan. Reimbursement policies are evolving, with payers increasingly favoring cost-effective, efficacious topical therapies to control healthcare costs associated with chronic skin conditions.
Price negotiations and formulary placements significantly influence market access and adoption rates for XERAC AC.
Revenue Projections and Market Penetration
Based on current market trends, an initial conservative market share of 2-3% in the global psoriasis and dermatitis treatment markets can be expected in the first year post-launch, gradually increasing to 8-10% by year five. Adoption rates are primarily driven by:
- Physician acceptance: Clinical trial data demonstrating superior efficacy.
- Patient adherence: Enhanced formulation promoting compliance.
- Pricing strategies: Competitive pricing to facilitate formulary inclusion.
Assuming a stabilization of the global addressable market at USD 4-6 billion annually for topical psoriasis and dermatitis treatments, XERAC AC could generate revenues in the range of USD 80 million to USD 600 million by year five, contingent on market share capture and pricing strategy.
Price Projection Analysis
Initial Launch Pricing
XERAC AC's initial pricing is projected at USD 250-350 per tube (30g), aligned with the premium segment due to its novel formulation and targeted efficacy. This positions it competitively against existing corticosteroids, which typically range from USD 150-300 per tube [2].
Pricing Dynamics Over Five Years
- Year 1: Competitive premium positioning at USD 300 per tube.
- Years 2-3: Slight reduction to USD 275-290 per tube to improve market penetration.
- Years 4-5: Further price adjustment to USD 250-270 per tube, influenced by increased competition, generic entries, and payer negotiations.
Factors Influencing Price Trends
- Generic/Copycat Entry: If patent exclusivity ends, generic versions could reduce prices by 40-60%.
- Market Competition: Introduction of competing formulations may necessitate price discounts.
- Pricing Pressure from Payers: Payers may negotiate for lower prices to control costs, influencing manufacturer strategies.
- Manufacturing Costs: Advances in manufacturing could reduce costs, allowing for flexible pricing.
Regulatory and Market Risks
Potential hurdles include:
- Regulatory Delays: Extended approval timelines could impact launch and revenue expectations.
- Reimbursement Challenges: If reimbursement policies favor lower-cost alternatives, pricing could be pressured downward.
- Market Acceptance: Prescriber preferences may favor established therapies unless XERAC AC demonstrates clear advantages.
Strategic Recommendations
- Value-Based Pricing: Emphasize clinical benefits and safety profile to justify premium pricing.
- Cost Optimization: Streamline manufacturing to maintain healthy margins amid competitive pressures.
- Market Education: Invest in clinician education to promote adoption based on efficacy and safety.
- Early Access Programs: Facilitate initial adoption and collect real-world data to support reimbursement negotiations.
Key Takeaways
- The global dermatology market for topical treatments is poised for consistent growth, with innovative agents like XERAC AC occupying a premium niche.
- Initial pricing is expected in the USD 250-350 range per tube, with gradual adjustments based on market dynamics.
- Revenue projections suggest potential for USD 80 million to over USD 600 million in annual sales within five years, assuming successful market penetration.
- Competitive pressures, patent timelines, and payer policies will significantly influence long-term pricing and market share.
- Strategic focus on clinical differentiation and cost management will be critical for maximizing value.
FAQs
1. What makes XERAC AC unique among topical therapies?
XERAC AC features a novel formulation that enhances skin absorption and targets specific inflammatory pathways, offering faster relief and improved safety compared to traditional corticosteroids.
2. How does the current competitive landscape affect XERAC AC’s market entry?
Existing therapies like corticosteroids and calcineurin inhibitors are entrenched, so XERAC AC must demonstrate clear advantages in efficacy, tolerability, or patient adherence to gain market traction.
3. What are the primary factors influencing XERAC AC’s price over the next five years?
Regulatory factors, market competition, payer negotiations, and patent status predominantly drive pricing adjustments.
4. How might patent expiry impact XERAC AC’s future revenue?
Patent expiration typically leads to generic entry, which could reduce prices by up to 60%, necessitating strategic shifts to sustain revenue and market share.
5. What strategies could accelerate XERAC AC’s adoption?
Investing in clinical trials to showcase efficacy, engaging physicians through education, and establishing favorable reimbursement agreements are vital for rapid uptake.
References
[1] Global Market Insights. "Dermatology Therapeutics Market Size & Share, 2022-2028."
[2] IQVIA Reports. "Topical Dermatological Products Pricing Analysis," 2023.
More… ↓
