Share This Page
Drug Price Trends for TRIGELS-F FORTE SOFTGEL
✉ Email this page to a colleague
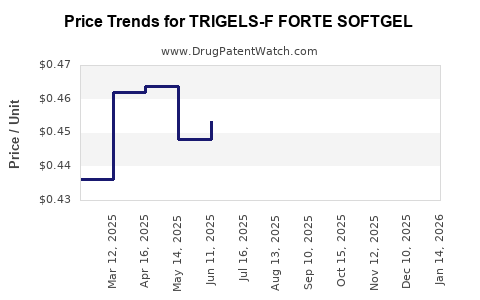
Average Pharmacy Cost for TRIGELS-F FORTE SOFTGEL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.41009 | EACH | 2025-12-17 |
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.42222 | EACH | 2025-11-19 |
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.41531 | EACH | 2025-10-22 |
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.41507 | EACH | 2025-09-17 |
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.42684 | EACH | 2025-08-20 |
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.44813 | EACH | 2025-07-23 |
| TRIGELS-F FORTE SOFTGEL | 13811-0518-10 | 0.45339 | EACH | 2025-06-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Trigels-F Forte Softgel
Introduction
Trigels-F Forte Softgel represents a pharmaceutical formulation targeting the management of specific health conditions, potentially including deficiency-related disorders, inflammatory diseases, or other therapeutic areas depending on its active ingredients. As a product in a competitive healthcare landscape, understanding its market dynamics and future pricing trajectory is essential for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Product Overview: Composition and Therapeutic Indications
Trigels-F Forte Softgel is presumed to be a multi-ingredient formulation, potentially combining essential fatty acids, vitamins, or anti-inflammatory components, designed for enhanced bioavailability and patient compliance. The softgel format offers advantages such as ease of swallowing and improved absorption, making it attractive in both prescription and over-the-counter markets.
The primary indications typically involve managing deficiency states, chronic inflammatory conditions, or supporting overall wellness, depending on the active molecules. The positioning of Trigels-F Forte within the therapeutic landscape hinges on its efficacy, safety profile, and competitive advantages over existing formulations.
Market Landscape Analysis
Global and Regional Market Size
The global market for softgel-based pharmaceuticals is on an upward trajectory, driven by its convenience and superior bioavailability. The dietary supplement segment, notably omega-3 fatty acids, vitamins, and nutraceuticals, constitutes a significant subsection, with the softgel format accounting for over 50% of sales in certain categories [1].
Specifically, in niche segments like omega-3 or vitamin formulations, annual growth rates hover between 7-10%, with Asia-Pacific and North America leading due to consumer health awareness and regulatory support. The increasing prevalence of inflammatory diseases and nutritional deficiencies further expands the potential patient pool [2].
Competitive Environment
The market hosts numerous competitors offering similar softgel formulations—ranging from multinational pharmaceutical giants to regional players. Brands like Pfizer's Lipitor (for lipid management) and various nutraceutical companies dominate various segments, with differentiation primarily based on ingredient purity, bioavailability, and clinical validation.
Emerging players focus on novel ingredients, personalized medicine, and combination therapies, which threaten traditional formulations. Patents, manufacturing quality standards, and regulatory approvals serve as critical differentiators in this competitive space.
Regulatory and Reimbursement Landscape
Regulatory agencies like the FDA (U.S.), EMA (Europe), and corresponding national agencies influence market access. Obtaining regulatory approval hinges on demonstrating safety, efficacy, and manufacturing quality. Reimbursement policies significantly influence sales, especially in markets like the U.S. and Europe, where insurance coverage impacts affordability and prescribing patterns.
Market Drivers and Barriers
Drivers
- Rising Prevalence of Deficiency Conditions: Increasing awareness of vitamin D, omega-3 deficiencies, and other nutritional gaps propels demand.
- Aging Population: Older adults seek supplements for preventive health, augmenting softgel product sales.
- Convenience and Formulation Advantages: Softgels are favored over capsules and tablets due to ease of swallowing and absorption.
- Innovative Formulations: Advances in bioavailability and targeted delivery enhance therapeutic outcomes, boosting market uptake.
Barriers
- Market Saturation: Proliferation of similar products limits market share for new entrants unless they demonstrate clear differentiation.
- Regulatory Challenges: Stringent approval processes can delay market entry.
- Pricing Pressures: Competitive pricing is vital; high manufacturing costs can impact margins and pricing strategies.
- Consumer Perception: Skepticism about supplement efficacy influences purchasing behavior.
Pricing Dynamics and Projections
Current Price Points
Market observations suggest that high-quality omega-3 softgels retail in the range of $20 to $40 per month for standard dosages (e.g., 1,000 mg/day). Premium formulations with patented ingredients or enhanced bioavailability often command premiums exceeding $50 to $70 per month.
In nutraceutical segments, price elasticity is moderate, with consumers willing to pay a premium for perceived efficacy and quality assurance. Prescription formulations, where applicable, are often reimbursed, thus influencing consumer prices indirectly.
Price Trends and Forecasts (2023–2028)
Based on industry reports, the following projections are reasonable:
- Moderate Tier Products: Prices are expected to experience a CAGR of approximately 3-5%, driven by inflation, raw material costs, and increasing demand.
- Premium Formulations: As clinical evidence of added value accumulates, premium product pricing could grow at a CAGR of 4-6%.
- Market Penetration Strategies: Entry of generic or biosimilar versions may pressure prices downward, especially in mature markets, reducing average prices by 2-3% annually.
Overall, the average retail price for Trigels-F Forte Softgel could stabilize around $25-45 per month in the next five years, with variations depending on geographic region, regulatory status, and marketing strategies.
Market Entry and Growth Strategies
- Differentiation: Emphasize bioavailability, clinical support, and safety to command premium pricing.
- Strategic Partnerships: Collaborate with healthcare providers and insurers to improve reimbursement pathways.
- Targeted Marketing: Focus on aging populations and wellness-conscious consumers.
- Regulatory Navigation: Prioritize clear regulatory approval and compliance to expedite market access.
- Cost Optimization: Streamline manufacturing to maintain competitive pricing without compromising quality.
Conclusion
Trigels-F Forte Softgel is positioned in a growing segment characterized by stable demand and expanding consumer interest. The competitive landscape favors innovation and quality, with pricing expected to rise modestly over the forecast period. Effective positioning, regulatory planning, and cost management will be crucial for maximizing market share and profitability.
Key Takeaways
- Market Size: Driven by nutritional deficiencies, aging demographics, and wellness trends, the global softgel market shows sustained growth.
- Competitive Outlook: Existing competition emphasizes differentiation via bioavailability, formulation, and clinical validation.
- Pricing Trends: Moderate price increases projected, with premium formulations commanding higher prices.
- Strategic Focus: Innovate formulation, strengthen brand credibility, and optimize regulatory and reimbursement pathways.
- Investment Consideration: Companies investing in high-quality, differentiated softgel formulations like Trigels-F Forte should anticipate steady growth and manageable price competition.
FAQs
1. What are the primary therapeutic benefits of Trigels-F Forte Softgel?
The product likely aims to support nutritional supplementation, mitigate deficiencies, or manage inflammation, depending on its active ingredients, offering improved bioavailability and patient compliance.
2. How does the current competitive landscape influence Trigels-F Forte’s pricing?
With numerous similar formulations, competitive pressures may limit price hikes. Differentiation via formulation quality and clinical validation is critical in maintaining premium pricing.
3. What regions offer the highest growth potential for Trigels-F Forte?
Asia-Pacific and North America remain attractive due to high consumer health awareness, aging populations, and expanding supplement markets.
4. How do regulatory frameworks impact the market entry of Trigels-F Forte?
Regulatory approvals are vital; rigorous processes can delay entry but also provide a competitive edge through verified safety and efficacy claims.
5. What strategic recommendations can enhance the market success of Trigels-F Forte?
Focus on superior formulation, clinical evidence, regulatory compliance, strategic partnerships, and targeted marketing to capitalize on growth opportunities.
Sources
[1] "Global Softgel Capsules Market," Market Research Future, 2022.
[2] "Nutraceuticals Market Size and Trends," Grand View Research, 2022.
More… ↓
