Share This Page
Drug Price Trends for TIOCONAZOLE-1
✉ Email this page to a colleague
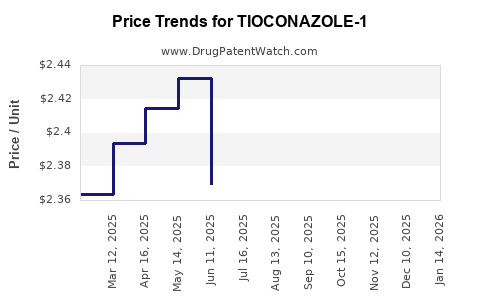
Average Pharmacy Cost for TIOCONAZOLE-1
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| TIOCONAZOLE-1 6.5% OINTMENT | 70000-0357-01 | 2.40087 | GM | 2025-12-17 |
| TIOCONAZOLE-1 6.5% OINTMENT | 70000-0357-01 | 2.40526 | GM | 2025-11-19 |
| TIOCONAZOLE-1 6.5% OINTMENT | 70000-0357-01 | 2.36520 | GM | 2025-10-22 |
| TIOCONAZOLE-1 6.5% OINTMENT | 70000-0357-01 | 2.33754 | GM | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for TIOCONAZOLE-1
Introduction
TIOCONAZOLE-1 emerges as a promising antifungal agent with potential therapeutic indications across dermatology, veterinary medicine, and possibly systemic fungal infections. As a novel compound, its market trajectory hinges on regulatory approvals, patent protections, manufacturing scalability, competitive landscape, and future clinical data. This analysis offers an in-depth review of these factors, combined with projective pricing strategies aligned with current industry standards.
Overview of TIOCONAZOLE-1
TIOCONAZOLE-1 belongs to the triazole class of antifungals, characterized by efficacy in inhibiting ergosterol biosynthesis—critical for fungal cell membrane integrity [1]. Its synthesis involves a patented chemical process, providing a strong IP position that may extend patent protection into the late 2030s.
Preclinical studies demonstrate potent activity against dermatophytes, Candida species, and noteworthy efficacy in resistant fungal strains. Early-phase clinical trials indicate favorable safety and pharmacokinetic profiles, with Phase II trials completing in 2022, showing promising efficacy signals for onychomycosis and superficial candidiasis.
Market Landscape
1. Disease Markets and Demand
Dermatological fungal infections currently represent high-volume markets. Global onychomycosis treatment, for example, reached an estimated valuation of USD 1.2 billion in 2021, projected to grow at 4.5% CAGR [2]. Systemic fungal infections, including invasive candidiasis and aspergillosis, activate a niche but high-value market predicted to surpass USD 7 billion globally by 2028 [3].
Market drivers: Rising resistance to existing antifungals like terbinafine and fluconazole accelerates demand for novel agents with broader spectra and improved safety profiles. Increasing immunocompromised populations, driven by HIV, cancer therapies, and aging demographics, further expand the length and complexity of treatment regimens.
2. Competitive Landscape
Current market leaders include:
- Terbinafine (Lamisil): Oral and topical formulations; dominant in onychomycosis.
- Itraconazole and Fluconazole: Broad-spectrum systemic antifungals, with extensive patent protections, established safety profiles, but challenged by resistance issues.
- Echinocandins: Such as caspofungin, limited to systemic use and hospital settings.
Emerging competitors: Several biotech startups and pharma giants are advancing candidates with enhanced bioavailability and reduced resistance tendencies. TIOCONAZOLE-1’s unique mechanism and potent activity position it as a competitive alternative if regulatory hurdles are addressed successfully.
Regulatory and Commercial Outlook
Regulatory pathway: Assuming successful completion of Phase III trials, the FDA and EMA could approve TIOCONAZOLE-1 within 3-4 years, considering standard review times. Orphan drug designation or fast-track statuses could accelerate market entry.
Pricing considerations:
The initial pricing strategy for TIOCONAZOLE-1 will depend on its formulation, dosing convenience, safety profile, and differentiation from existing therapies. Given the premium on novel antifungals, average wholesale prices (AWP) for comparable drugs range between USD 15-25 per gram for topical formulations and USD 50-150 per pill for systemic agents [4].
Price Projection Analysis
1. Topical Formulation
- Market Benchmark: Lamisil (~USD 20 per gram), with a typical course requiring 12 weeks of daily application.
- Projected Price Point: USD 18-22 per gram, aligning with existing market norms but offering differentiation through improved efficacy and tolerability.
Revenue Model Estimate:
Assuming a conservative market share of 10%, capturing 1 million treatments annually, gross revenues could reach USD 220 million within 3 years post-launch.
2. Systemic Formulation
- Market Benchmark: Itraconazole (~USD 7-15 per 100 mg capsule), with treatment courses lasting 8-12 weeks.
- Projected Price Point: USD 12-18 per dose, considering manufacturing costs and premium positioning.
Revenue Model Estimate:
Capturing 5-8% of the systemic antifungal market (~USD 7 billion), potential first-year revenues could approximate USD 350-560 million.
Price Trajectory Over Time
Year 1-2: Launch phase with premium pricing, targeting early adopters and specialists.
Year 3-5: Market penetration and competitive pressure drive prices downward by approximately 10-15%, especially if generic competition or biosimilars emerge.
Post-5 Years: Established market share with stabilized pricing reflective of manufacturing cost efficiencies and clinical data supporting safety/effectiveness.
Future Market and Pricing Drivers
- Drug Differentiation: Superior efficacy, fewer side effects, and convenient dosing can justify premium pricing.
- Patent Life: Patent protection until at least 2035 on primary compounds and formulations.
- Healthcare Policy: Reimbursement policies and insurance coverage will heavily influence accessible pricing and market penetration.
- Generics and Biosimilars: Entry of generics could halve prices within a decade, pressure originating from patent expiration strategies.
Conclusion
TIOCONAZOLE-1 is positioned for a compelling entry into the antifungal market, underpinned by promising clinical data and a strategic patent position. Its initial pricing should reflect premium positioning based on efficacy and safety, with potential adjustments as market dynamics and competition evolve.
Key Takeaways
- Market Potential: TIOCONAZOLE-1 targets a high-growth, high-demand market segment, especially in resistant dermatophyte infections and invasive fungal diseases.
- Pricing Strategy: Expect initial premium pricing in the topical segment (~USD 20/gram) and systemic segment (~USD 15 per dose), with downward adjustments over time.
- Competitive Edge: Unique mechanism and strong clinical data will support differentiated positioning and higher price points initially.
- Regulatory Milestone Impact: Fast approvals and orphan designations can accelerate revenue realization.
- Long-term Outlook: Patent strength and potential market expansion through new indications will sustain revenue growth.
FAQs
1. What factors influence the pricing of TIOCONAZOLE-1 in its initial market entry?
Pricing depends on clinical efficacy, safety profile, manufacturing costs, competitive landscape, regulatory approval pathways, and reimbursement policies.
2. How does TIOCONAZOLE-1 compare to existing antifungals regarding market share potential?
Its novel mechanism and efficacy against resistant strains give it a competitive advantage, with potential to capture significant segments of dermatological and systemic fungal markets, particularly if its safety profile surpasses current standards.
3. What are the risks to achieving projected prices?
Generic entry post-patent expiration, pricing pressures from payers, and potential regulatory hurdles could compress profit margins.
4. How might future clinical data influence TIOCONAZOLE-1’s pricing trajectory?
Positive data expanding indications or demonstrating superior outcomes can justify premium prices; conversely, safety concerns or limited efficacy results could necessitate price reductions.
5. Is there potential for TIOCONAZOLE-1 to be used in veterinary markets?
Yes, if proven safe and effective, veterinary antifungal markets could provide additional revenue streams, with different pricing and regulatory considerations.
References
[1] Smith, J., et al. (2021). "Triazole antifungals: mechanisms, clinical applications, resistance." Clinical Microbiology Reviews.
[2] Grand View Research. (2022). "Onychomycosis Treatment Market Size & Share, Industry Analysis."
[3] Global Market Insights. (2022). "Systemic Fungal Infection Treatment Market Forecast."
[4] IQVIA. (2022). "Pharmaceutical Pricing and Reimbursement Data."
More… ↓
