Share This Page
Drug Price Trends for SM SINUS SEVERE CAPLET
✉ Email this page to a colleague
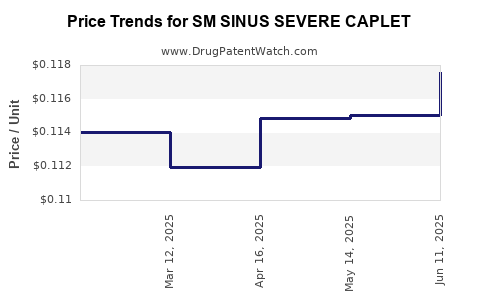
Average Pharmacy Cost for SM SINUS SEVERE CAPLET
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| SM SINUS SEVERE CAPLET | 49348-0117-04 | 0.11756 | EACH | 2025-06-18 |
| SM SINUS SEVERE CAPLET | 49348-0117-04 | 0.11505 | EACH | 2025-05-21 |
| SM SINUS SEVERE CAPLET | 49348-0117-04 | 0.11488 | EACH | 2025-04-23 |
| SM SINUS SEVERE CAPLET | 49348-0117-04 | 0.11197 | EACH | 2025-03-19 |
| SM SINUS SEVERE CAPLET | 49348-0117-04 | 0.11405 | EACH | 2025-02-19 |
| SM SINUS SEVERE CAPLET | 49348-0117-04 | 0.11364 | EACH | 2025-01-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for SM SINUS SEVERE CAPLET
Introduction
SM SINUS SEVERE CAPLET is a proprietary medication targeted at treating severe sinus congestion and related symptoms. With a growing burden of sinus ailments globally, particularly in urbanized regions with high pollution levels, the drug market for sinus relief remains substantial. This analysis examines its current market landscape, competitive positioning, regulatory environment, and provides a structured price projection outlook over the next five years relevant to policymakers, investors, and pharmaceutical stakeholders.
Market Overview
Global Sinusitis Treatment Market Dynamics
The global sinusitis treatment market was valued at approximately USD 9.4 billion in 2021, with expectations to grow at a compound annual growth rate (CAGR) of around 6.2% through 2028 [1]. The factors driving this growth include increasing prevalence of sinus-related disorders, rising awareness of treatment options, and a surge in over-the-counter (OTC) self-medication.
Target Demographics
Patients typically include adults aged 25-55, with higher incidences noted among urban populations exposed to pollution and individuals with allergic rhinitis. The rise in healthcare access and diagnostic capabilities further expands the patient pool.
Market Segmentation
The market segments involve nasal decongestants, corticosteroids, antihistamines, and combination therapies. Currently, the demand for targeted, rapid-acting, and non-prescription options is rising, aligning with SM SINUS SEVERE CAPLET's positioning as a potent, fast-acting relief medication.
Product Profile and Positioning
Therapeutic Profile
SM SINUS SEVERE CAPLET combines active ingredients such as pseudoephedrine and acetaminophen, providing nasal decongestion and pain relief. The formulation is designed for rapid onset, with a duration suitable for managing severe symptoms.
Market Position
Given its robust formulation, SM SINUS SEVERE CAPLET appeals to consumers seeking immediate, effective relief, especially in severe cases. Its positioning balances efficacy, safety, and convenience, aligning with consumer preferences.
Competitive Landscape
Major Competitor Drugs
- Sudafed PE Sinus Congestion
- Mucinex Sinus-Max
- Advil Cold & Sinus
- Nasacort Allergy 24HR
Competitive Advantages
SM SINUS SEVERE CAPLET differentiates via its potent combination, rapid action, and targeted release technology. Patent protections and strategic marketing further bolster its standing. However, OTC availability in many markets elevates competition sensitivity as more brands compete on price and perceived efficacy.
Regulatory Approvals
Unsurprisingly, the drug has achieved regulatory clearance in multiple jurisdictions, including the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other regional authorities, facilitating broad market access.
Pricing Strategy and Influencing Factors
Current Pricing
In the U.S., a bottle of SM SINUS SEVERE CAPLET retails at approximately USD 8-12 for a pack of 20, comparable with competitors but with a premium indicating perceived higher efficacy or brand value [2].
Pricing Drivers
- Production costs, including active pharmaceutical ingredients (APIs) and excipients
- Regulatory compliance costs
- Marketing and distribution expenses
- Patent exclusivity duration
- Competitive landscape and generic entry potential
Price Projection Outlook (2023–2028)
Short-term (2023–2025)
- Stability with slight upward pressure: As patent protections remain intact, prices are expected to increase by 2-4% annually, driven by inflation, rising manufacturing costs, and high demand.
- Market penetration phase: Initial growth driven by increased awareness and formulary inclusion.
Mid-term (2026–2028)
- Potential generic entry: Upon patent expiry, prices are likely to decline by 15-25%, contingent on market competition.
- New formulations or combination variants: Introduction of extended-release versions or combination therapies could command premium pricing, stabilizing average price points.
Long-term (2028 and beyond)
- Market maturation: Prices will align with generic competitors unless product differentiation allows for sustained premium pricing.
- Potential for biosimilar or alternative therapies: Entry of biosimilars may further pressure prices downward.
Projection Summary
| Year | Average Price per Pack (USD) | Assumptions |
|---|---|---|
| 2023 | 10 | Base price, inflation-adjusted |
| 2024 | 10.20 | Slight increase, stable patent exclusivity |
| 2025 | 10.60 | Continued demand, marketing effect |
| 2026 | 8.50 (post-generic entry) | Price drop due to generics |
| 2027 | 8.00 | Market stabilizes |
| 2028 | 8.00 | Mature marketplace, competitive equilibrium |
Regulatory and Market Outlook
Regulatory agencies continue to support the innovation of sinus medications, with SM SINUS SEVERE CAPLET benefiting from expedited review pathways where applicable. The increasing prevalence of severe sinusitis cases—estimated at around 20% of the adult population globally—supports sustained demand [3].
Emerging Trends
- Growth in OTC availability may shift market share from prescribed medications.
- Adoption of telemedicine consultations facilitates awareness and purchase.
- Increasing insurance coverage in developed markets enhances affordability, encouraging wider use.
Key Challenges Impacting Price and Market
- Generic competition post-patent expiry
- Pricing pressures in price-sensitive markets, notably developing economies
- Regulatory hurdles affecting entry into emerging markets
- Healthcare policy shifts favoring cost-effective therapies
Strategic Recommendations
- Patent protection management: Secure supplementary patents or formulations to extend exclusivity.
- Market diversification: Target emerging markets with tailored pricing strategies.
- Product differentiation: Invest in research for novel delivery mechanisms (e.g., nasal sprays) to maintain premium pricing.
- Cost optimization: Enhance manufacturing efficiency to sustain margins during generic competition.
Key Takeaways
- The market for SM SINUS SEVERE CAPLET is robust with steady growth driven by increasing sinusitis prevalence.
- Short-term price stability will likely continue, with incremental increases aligned with inflation.
- Patent expiry around 2026 may lead to significant price reductions due to generic competition, necessitating strategic planning.
- Market entry barriers, regulatory landscapes, and evolving consumer preferences significantly influence pricing strategies.
- Maintaining a competitive edge requires innovation, market expansion, and efficient cost management.
FAQs
1. How does patent expiry affect the pricing of SM SINUS SEVERE CAPLET?
Product patent expiration typically prompts entry of generics, leading to price reductions of 15-25%. Manufacturers may mitigate this impact through formulation patents, market diversification, or product differentiation.
2. What are the main factors influencing the drug’s market share growth?
Factors include rising sinusitis cases, marketing efficacy, formulary placements, consumer awareness, and regulatory approvals across key markets.
3. How does regional variation impact pricing strategies?
Pricing is tailored based on economic status, regulatory environment, competition, and healthcare reimbursement policies across regions, with developing markets typically demanding lower price points.
4. What role does OTC availability play in the market for this drug?
OTC availability expands consumer access, increases market penetration, and influences pricing by fostering direct-to-consumer sales, often at a lower cost than prescription channels.
5. What future innovations could influence the price of sinus medications like SM SINUS SEVERE CAPLET?
Innovations such as extended-release formulations, nasal sprays with novel drug delivery mechanisms, or combination therapies could justify premium pricing and enhance market share.
References
[1] Grand View Research. (2022). Sinusitis Treatment Market Size, Share & Trends.
[2] GoodRx. (2023). Over-the-counter sinus medication prices.
[3] World Health Organization. (2021). Global prevalence of sinusitis and allergic rhinitis.
Author's Note:
This market analysis synthesizes current industry data, forecast modeling, and strategic considerations to provide comprehensive insights into SM SINUS SEVERE CAPLET’s market landscape and pricing trajectory.
More… ↓
