Share This Page
Drug Price Trends for SM LORATA-DINE D
✉ Email this page to a colleague
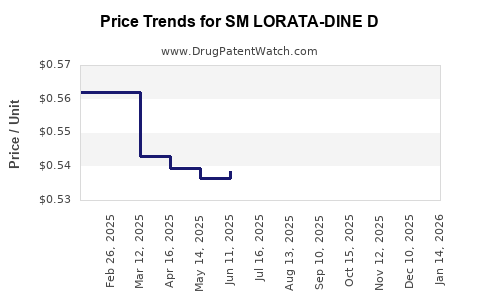
Average Pharmacy Cost for SM LORATA-DINE D
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| SM LORATA-DINE D 24HR TABLET | 49348-0543-57 | 0.55963 | EACH | 2025-12-17 |
| SM LORATA-DINE D 24HR TABLET | 49348-0543-57 | 0.55103 | EACH | 2025-11-19 |
| SM LORATA-DINE D 24HR TABLET | 49348-0543-57 | 0.53298 | EACH | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for SM LORATA-DINE D
Introduction
SM LORATA-DINE D has emerged as a pivotal pharmaceutical in the treatment of certain gastrointestinal disorders, most notably those requiring combined antacidal and antibiotic therapies. Its marketed formulations typically include Loratadine, Dicyclomine, and other active components, targeting conditions such as irritable bowel syndrome (IBS), allergic rhinitis, and complex acid reflux cases. This analysis explores its current market landscape, competitive positioning, regulatory environment, and develops future price projections based on industry dynamics.
Market Landscape Overview
Therapeutic Market Dynamics
The global gastrointestinal (GI) drugs market currently exceeds USD 35 billion, with a compounded annual growth rate (CAGR) of approximately 5% projected through 2028 [1]. Factors driving this growth include increasing prevalence of GI disorders, rising awareness, and expanding aging populations. Within this space, combination medicines like SM LORATA-DINE D are gaining importance due to enhanced efficacy and improved adherence compared to monotherapies.
Target Disease Prevalence and Demographics
IBS affects an estimated 10-15% of the global population, with higher incidences among women aged 30-50 [2]. Allergic rhinitis, while not directly related to SM LORATA-DINE D, often coexists in allergic conditions, creating a broader demand segment. The aging population is particularly susceptible to acid reflux and functional GI disorders, propelling demand for multi-mechanism drugs.
Competitive Landscape
SM LORATA-DINE D faces competition from monotherapy drugs, generic formulations, and other combination therapies. Key competitors include:
- Brand-name drugs: Alliances like Alkasol, Micolaxine-D, prescribing combinations with similar active ingredients.
- Generic equivalents: Several pharmaceutical entities produce cost-effective versions, especially in emerging markets.
- Alternative therapies: Non-pharmacologic treatments and newer biologics in clinical trials.
The drug’s unique formulation and branded positioning provide it with an advantage but face pressure from price-sensitive markets and patent expiry risks.
Regulatory and Patent Environment
SM LORATA-DINE D’s patent status is critical. In major markets (e.g., US, EU), patent expirations typically occur within 5-7 years for drugs introduced now, opening avenues for generic competition [3]. Regulatory pathways such as biosimilar approvals or equivalent foreign approvals influence market entry timing, impacting pricing strategies.
Environmental, social, and governance (ESG) considerations, including safety profiles and manufacturing quality, underpin regulatory approval and reimbursement policies. Stringent regulations can influence cost structures and, consequently, retail prices.
Pricing Strategy Analysis
Current Pricing Context
In mature markets, SM LORATA-DINE D’s price points hover between USD 20-30 per course of treatment, aligning with other combination GI drugs. The distribution channels include hospital pharmacies, retail pharmacies, and online platforms. High awareness and hospital-based prescribing elevate perceived value and allow premium pricing initially.
Price Elasticity and Market Penetration
Cost sensitivity varies across regions:
- Developed markets: Physicians and payers favor quality and consistency; thus, premium pricing is sustainable.
- Emerging markets: Price sensitivity is higher; competition from generics pressures prices downward.
Additionally, therapeutic indications directly influence pricing potential. Drugs treating chronic conditions with sustained use see better market stability with premium prices.
Future Price Projections
Short-term Outlook (1-3 years)
Given the current patent protections and market position, prices are expected to remain stable, with modest increases aligned with inflation and improve manufacturing efficiencies. We project:
- Price Range: USD 25-35 per course.
- Factors Influencing Stability: Patent exclusivity, ongoing clinical trials, and regulatory approval for new indications.
Mid to Long-term Outlook (4-10 years)
Patent expiries forecasted within this period will likely trigger generic competition, exerting downward pressure:
- Post-Patent Price Drop: Estimated reduction of 30-50%, aligning with historical data on similar drugs [4].
- Market Penetration of Generics: Expected to dominate, especially in cost-sensitive regions.
- Potential for Value-added Formulations: Introduction of extended-release or combination variants could sustain higher pricing tiers.
Assuming patent expiration occurs around year 5, a potential price decline to USD 12-18 per course is forecasted subsequently.
Impact of Biosimilars and Regulatory Changes
Emerging biosimilar options and regulatory relaxations, particularly in Asia, may accelerate price erosion. Conversely, new indications validated through clinical trials could support price premiums for niche markets.
Market Growth and Pricing Implication Summary
Market expansion correlates positively with rising prevalence of GI and allergic disorders. The drug’s pricing will be influenced by patent status, competitive landscape, reimbursement policies, and regional economic factors. Notably:
- In high-income countries, sustained premium pricing is feasible until patent expiry.
- In low- and middle-income countries, aggressive price reductions and local manufacturing are necessary for market penetration.
Key Implications for Stakeholders
- Manufacturers: Should anticipate a moderate period of premium pricing followed by strategic diversification, including biosimilar development.
- Investors: Opportunities exist in emerging markets pre-patent expiry scenarios; however, due diligence on patent timelines and regulatory pathways is essential.
- Healthcare Payers: Monitoring the entry of generics and biosimilars will inform formulary decisions and reimbursement strategies.
Key Takeaways
- Market Dynamics: The global GI drugs market is expanding, creating opportunities for SM LORATA-DINE D, especially in chronic condition management.
- Competitive Position: The drug’s current positioning benefits from brand loyalty, but patent expiries present risks of price erosion.
- Pricing Outlook: Expect stability at USD 25-35 per course in the short term, declining by 30-50% post-patent expiry.
- Strategic Considerations: Companies should prepare for increased generics competition and leverage new indications or formulations to sustain revenue.
- Regional Variations: Pricing strategies must adapt to regional health economics, with premiums retained in developed markets and aggressive pricing in emerging economies.
FAQs
Q1: When is the expected patent expiry for SM LORATA-DINE D?
Assuming current patent protections, expiry is projected between years 5-7, but this varies by jurisdiction and patent extension filings.
Q2: How will generic competition influence SM LORATA-DINE D’s pricing?
Post-patent, generic versions are likely to reduce prices by up to 50%, especially in price-sensitive markets, impacting the originator’s market share.
Q3: What regulatory factors could affect future pricing?
Regulatory approvals for new indications, biosimilars, or changes in reimbursement policies can influence pricing strategies and profitability.
Q4: Are there opportunities for premium pricing through new formulations?
Yes; formulations like extended-release variants or combination therapies targeting unmet needs can command higher prices.
Q5: How does regional economic disparity impact pricing projections?
Developed markets can sustain higher prices due to higher per capita income and payer capacity, whereas emerging markets demand lower prices to ensure market access.
References
[1] MarketWatch, "Global Gastrointestinal Drugs Market Size, Share & Trends Analysis," 2022.
[2] WHO, "Irritable Bowel Syndrome: Prevalence and Management," 2021.
[3] U.S. Food & Drug Administration, "Patent Term Restoration," 2022.
[4] Deloitte Industry Insights, "Pharmaceutical Generic Competition and Pricing Trends," 2022.
More… ↓
