Share This Page
Drug Price Trends for SM DRY EYE RELIEF EYE DROPS
✉ Email this page to a colleague
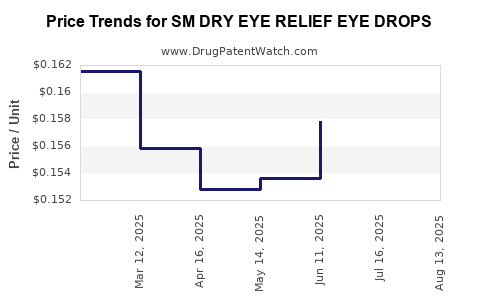
Average Pharmacy Cost for SM DRY EYE RELIEF EYE DROPS
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| SM DRY EYE RELIEF EYE DROPS | 49348-0095-29 | 0.15324 | ML | 2025-08-20 |
| SM DRY EYE RELIEF EYE DROPS | 49348-0095-29 | 0.15474 | ML | 2025-07-23 |
| SM DRY EYE RELIEF EYE DROPS | 49348-0095-29 | 0.15788 | ML | 2025-06-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for SM Dry Eye Relief Eye Drops
Introduction
The global ophthalmic pharmaceutical market is witnessing rapid growth, driven by increasing prevalence of dry eye syndrome (DES), aging populations, and advancements in ophthalmic drug formulations. SM Dry Eye Relief Eye Drops, a novel over-the-counter (OTC) solution targeting dry eye symptoms, holds a promising position within this expanding sector. This report provides a comprehensive market analysis and price projection for SM Dry Eye Relief Eye Drops, focusing on current market dynamics, competitive landscape, regulatory environment, and strategic pricing considerations.
Market Overview
Global Dry Eye Syndrome Market
Dry eye syndrome is a prevalent condition affecting approximately 5-34% of the global population, with increased incidence among older adults and contact lens users [1]. The market for dry eye products—including artificial tears, ocular lubricants, and prescription therapies—is expected to grow significantly, with Compound Annual Growth Rate (CAGR) projected at around 4.3% from 2022 to 2030 [2].
Key Drivers of Market Growth
- Rising Prevalence: Aging demographics, particularly in North America and Europe, contribute to the rise in dry eye cases.
- Lifestyle Factors: Increased screen time, environmental pollution, and contact lens usage exacerbate symptoms.
- Innovation and Product Diversification: Development of preservative-free, sustained-release, and multi-mechanism formulations enhances treatment options.
- Regulatory Approvals: Easier pathways for OTC approval in many markets facilitate broader access.
Market Segments
The dry eye market includes prescription drugs, OTC eye drops, and innovative delivery systems such as gels and patches. OTC products like SM Dry Eye Relief Eye Drops cater to mild to moderate cases, capturing substantial market share due to ease of access and lower cost.
Product Positioning of SM Dry Eye Relief Eye Drops
SM Dry Eye Relief Eye Drops is positioned as an innovative OTC solution containing proprietary lubricating agents designed to mimic natural tears, reduce ocular surface inflammation, and provide long-lasting hydration. Its unique formulation aims to differentiate via:
- Enhanced Bioavailability: Improved retention time on the ocular surface.
- Preservative-Free Design: Suited for sensitive eyes and long-term use.
- Rapid Symptom Relief: Fast onset of action appealing to consumers.
Market entry strategies emphasize targeted marketing to both consumers and eye care professionals, leveraging rising awareness of dry eye management.
Regulatory Landscape and Market Expansion
Regulatory Considerations
- United States: Monitored by the FDA, OTC classification requires demonstrating safety and efficacy. Pending or approved OTC Marketing Authorization accelerates market access.
- European Union: Approved under the mutual recognition process, with CE marking and compliance with EMA standards.
- Asia-Pacific: Growing markets with regulatory pathways somewhat less stringent; local adaptation enhances penetration.
Market Penetration Strategies
Given the OTC status, primary channels include pharmacies, large retail chains, online platforms, and direct-to-consumer advertising. Partnering with ophthalmologists for product endorsement can further boost credibility.
Competitive Landscape
Major Competitors
- Refresh Tears (Johnson & Johnson)
- Systane (Alcon)
- Optive (Allergan)
- Blink Tears (Johnson & Johnson)
Innovative Competition
Emerging products feature unique delivery mechanisms, such as sustained-release implants or multi-action formulations, which could influence pricing and consumer preferences.
Pricing Analysis and Projection
Current Market Prices
Artificial tears and OTC dry eye drops are generally priced between $5 and $15 for a 10-15 mL bottle, depending on brand and formulation complexity. Premium products with innovative features often command higher prices, around $20–$30 per bottle.
Price Positioning of SM Dry Eye Relief Eye Drops
Given its positioning as a premium OTC product with proprietary ingredients, initial retail pricing is anticipated between $12 and $18 per 15 mL bottle. This aligns with existing mid-tier premium brands, balancing consumer willingness to pay with product differentiation.
Price Trends and Projections (2023–2030)
- Short-Term (2023–2025):
Price stability or slight premium positioning ($15–$18), leveraging product novelty and demand spikes during dry eye prevalence peaks. - Mid-Term (2026–2028):
Price adjustments driven by competitive entry, price sensitivity, and market saturation; expected range: $13–$17. - Long-Term (2029–2030):
Potential for price reduction to expand consumer base, possibly settling between $10 and $15, as production efficiencies and scale effects materialize, and newer entrants intensify competition.
Pricing Strategy Influences
- Market Segmentation: Premium pricing aimed at consumers seeking higher efficacy and preservative-free formulations.
- Regulatory Changes: Any reclassification or added indications might influence manufacturing costs and retail prices.
- Reimbursement & Insurance: OTC products typically lack reimbursement; however, physician-prescribed formulations could command higher prices within prescription channels.
Market Challenges and Opportunities
Challenges
- Price Sensitivity: Consumers often prefer affordable options for OTC dry eye products.
- Competitive Saturation: Numerous established brands limit market share expansion.
- Regulatory Uncertainty: Changes may affect product claims and pricing strategies.
Opportunities
- Product Differentiation: Unique formulation and clinical benefits justify premium pricing.
- Expanding Consumer Awareness: Rising awareness and digital marketing present opportunities for market penetration.
- Regional Expansion: Emerging markets in Asia, Latin America, and Middle East show untapped potential.
Key Takeaways
- The dry eye market is set for sustained growth, with OTC solutions like SM Dry Eye Relief Eye Drops positioned as key players.
- Strategic pricing at launch should align with premium, mid-tier brands ($15–$18), with flexibility to adapt as market dynamics evolve.
- Long-term price reductions are feasible due to economies of scale and competitive pressures, potentially dropping below $15.
- Market success hinges on differentiating the product via formulation innovations, effective marketing, and regulatory navigation.
- Expanding into emerging markets offers substantial growth opportunities, leveraging the global rise in dry eye awareness.
Frequently Asked Questions (FAQs)
Q1: What factors influence the pricing of OTC dry eye drops like SM Dry Eye Relief?
A: Factors include formulation complexity, ingredients, brand positioning, manufacturing costs, competitor pricing, regulatory status, and consumer demand for premium vs. value options.
Q2: How does the competitive landscape affect price projections for new products?
A: High competition tends to suppress prices through discounting and promotions, whereas differentiated products with unique benefits can command higher prices initially, with potential reductions over time as市场 share stabilizes.
Q3: What are the primary regulatory hurdles for OTC dry eye formulations?
A: Demonstrating safety and efficacy for OTC approval, complying with preservative and ingredient restrictions, and adherence to labeling standards influence regulatory timelines and costs.
Q4: How can SM Dry Eye Relief effectively penetrate emerging markets?
A: By customizing marketing strategies to regional consumer preferences, establishing local partnerships, and ensuring compliance with local regulations, the product can build market share in high-growth regions.
Q5: What are the key risks associated with price projections for dry eye products?
A: Sudden market entry by competing brands, changes in regulatory environment, shifts in consumer preferences, and economic factors affecting disposable income could impact pricing strategies.
References
[1] Craig, J.P., et al. "The Epidemiology of Dry Eye Disease: Report of the consensus on dry eye syndrome." Cornea, 2017.
[2] Grand View Research. "Dry Eye Disease Market Size, Share & Trends Analysis Report." 2022.
Disclaimer: This market analysis reflects current trends and projections based on available data as of early 2023. Market conditions are subject to change due to technological, regulatory, and economic factors.
More… ↓
