Share This Page
Drug Price Trends for SENNA LAX
✉ Email this page to a colleague
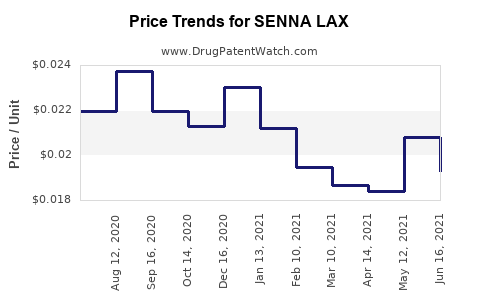
Average Pharmacy Cost for SENNA LAX
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| SENNA LAXATIVE 8.6 MG TABLET | 70000-0447-03 | 0.02325 | EACH | 2025-01-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for SENNA LAX
Introduction
SENNA LAX, a widely used over-the-counter (OTC) laxative, contains natural senna extract as its active ingredient, and is formulated to alleviate occasional constipation. Its popularity stems from its efficacy, minimal side effects, and affordability. As regulatory frameworks evolve and consumer preferences shift toward natural and herbal remedies, understanding the current market landscape and forecasting future pricing mechanisms are crucial for stakeholders, including manufacturers, investors, and healthcare policymakers.
Market Overview
Global Market Demand
The global laxative market, valued at approximately USD 4 billion in 2022, is projected to reach USD 5.7 billion by 2028, growing at a CAGR of around 6.2% [1]. Senna-based products account for a significant share within herbal and OTC laxatives, driven by consumer preference for natural alternatives.
The rise in gastrointestinal disorders, aging populations, and increased awareness of digestive health support sustained demand for senna-based laxatives like SENNA LAX. Additionally, the COVID-19 pandemic heightened health consciousness and self-medication tendencies, further amplifying OTC sales.
Regional Market Variability
North America dominates the market, bolstered by high consumer awareness, extensive OTC access, and a substantial geriatric population. Europe follows, with growing herbal remedy adoption. The Asia-Pacific region exhibits rapid growth, attributed to expanding healthcare infrastructure, increased urbanization, and traditional medicine integration.
Regulatory Landscape
SENNA LAX products are regulated as OTC drugs in the United States (by the FDA) and similar agencies worldwide, with permissible dosage and labeling restrictions. Concerns over potential laxative overuse and dependency have led to tighter regulations in some markets, influencing manufacturing and marketing strategies.
Competitive Landscape
Major Players
Key manufacturers include:
- Bayer AG: With its Phospho-Soda line, Bayer remains influential.
- Colgate-Palmolive: Distributes Senokot, a leading senna-based OTC laxative.
- GlaxoSmithKline (GSK): Offers diverse gastrointestinal products.
- Nature’s Way and other herbal supplement companies are also active in the herbal laxative sector.
These firms focus on product differentiation via formulation improvements, dosage forms (tablets, teas, liquids), and branding emphasizing natural ingredients.
Market Entry Barriers
Regulatory compliance, product safety concerns, and established brand loyalty pose high entry barriers for new incumbents. Moreover, patent protections for specific formulations or delivery mechanisms hinder competitive innovation.
Pricing Dynamics
Current Pricing Analysis
The retail price of SENNA LAX varies globally but remains relatively affordable in the OTC segment:
- United States: Approximately USD 4–8 per bottle (containing 50–100 tablets).
- European Markets: EUR 3–7 per package.
- Asia-Pacific: Similar or slightly lower, reflecting regional manufacturing and distribution costs.
Price variations depend on packaging size, brand reputation, and formulation (e.g., herbal blends vs. pure senna extracts).
Pricing Factors
- Manufacturing costs: Raw herbal material sourcing, processing, quality control.
- Regulatory compliance: Clinical safety data, labeling standards, and packaging requirements.
- Market positioning: Premium herbal or organic labels command higher prices.
- Distribution channels: Pharmacy vs. retail vs. online platforms.
Price Trends
Over the last five years, prices have remained relatively stable, with minor fluctuations driven by raw material cost variations and regulatory changes. Pandemic-induced supply chain disruptions temporarily increased costs, but market prices quickly stabilized owing to competitive pressures.
Future Price Projections
Influencing Factors
-
Regulatory Tightening: Stricter safety standards and possible restrictions on senna’s permissible daily doses could reduce supply or increase compliance costs, potentially raising retail prices.
-
Raw Material Availability: Climate change and farming conditions impact senna leaf harvests. Scarcity may elevate raw material prices, cascading into retail costs.
-
Consumer Trends: Growing demand for organic and herbal OTC products could elevate willingness to pay premium prices in developed markets.
-
Innovation and Formulation: Development of sustained-release formulations or combination products could command higher pricing.
-
Market Competition: Increased entry of new players or alternative herbal laxatives could intensify price competition, exerting downward pressure.
Forecast Assumptions
Based on current trends, the following can be posited:
- Short-term (1-2 years): Prices are likely to remain stable, with minimal fluctuations, barring raw material shortages or regulatory actions.
- Medium-term (3-5 years): Prices might marginally increase (by 3–5%) due to ingredient cost escalation and reformulation mandates.
- Long-term (5+ years): Significant shifts depend on technological innovations, regulatory environment evolution, and consumer preferences; potential for higher premium pricing for organic or specialty formulations exists.
Strategic Insights for Stakeholders
- Manufacturers should invest in sustainable sourcing of senna leaves and innovative formulations to mitigate raw material risks.
- Investors should monitor regulatory trends closely, as potential restrictions could impact supply and profitability.
- Marketers can capitalize on the natural, herbal appeal to command premium pricing tiers in affluent markets.
- Policymakers must balance consumer safety with access, considering tightening regulations that could affect market prices.
Key Takeaways
- The global SENNA LAX market remains robust, driven by increasing gastrointestinal health awareness and a preference for herbal remedies.
- Pricing stability is expected in the short term, with modest increases projected over the medium to long term due to rising raw material costs and innovation.
- Competitive landscape is characterized by well-established players, high entry barriers, and a continuous emphasis on natural and organic branding.
- Supply chain resilience and regulatory compliance are critical factors affecting future pricing strategies.
- Stakeholders should adopt sustainable sourcing and innovative product development to maintain competitiveness and optimize profitability.
FAQs
1. What factors influence the retail price of SENNA LAX?
Retail prices are affected by raw material costs, manufacturing expenses, regulatory compliance, branding efforts, formulation complexity, and distribution channel margins.
2. How might regulatory changes impact SENNA LAX pricing?
Stricter safety standards or dosage restrictions could increase manufacturing costs or reduce supply, potentially elevating prices. Conversely, deregulation could lower costs and prices.
3. Is there a trend towards natural or organic formulations in SENNA LAX?
Yes, consumer preferences favor natural, organic, and herbal products, leading manufacturers to develop premium formulations that can command higher prices.
4. What regions present the greatest growth opportunities for SENNA LAX?
The Asia-Pacific region exhibits high growth potential due to increasing urbanization, disposable incomes, and traditional medicine integration.
5. What risks could threaten the stability of SENNA LAX prices?
Raw material scarcity, new regulatory restrictions, market entry of substitutes, and supply chain disruptions pose significant risks to price stability.
References
- Market Research Future. (2022). "Laxative Market Analysis & Forecast."
More… ↓
