Share This Page
Drug Price Trends for PUREVIT DUALFE PLUS CAPSULE
✉ Email this page to a colleague
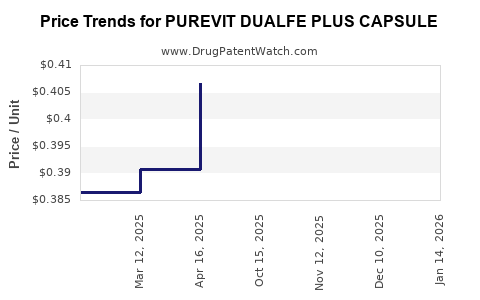
Average Pharmacy Cost for PUREVIT DUALFE PLUS CAPSULE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| PUREVIT DUALFE PLUS CAPSULE | 59088-0112-66 | 0.38689 | EACH | 2025-12-17 |
| PUREVIT DUALFE PLUS CAPSULE | 59088-0112-66 | 0.38412 | EACH | 2025-11-19 |
| PUREVIT DUALFE PLUS CAPSULE | 59088-0112-66 | 0.37967 | EACH | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for PUREVIT DUALFE PLUS CAPSULE
Introduction
PUREVIT DUALFE PLUS CAPSULE is a pharmaceutical product primarily marketed for its dual-action formulation, emphasizing vitamin D supplementation coupled with other health benefits. As interest in nutritional supplements and fortified medications escalates globally, understanding the market potential and pricing strategies for PUREVIT DUALFE PLUS is essential for industry stakeholders, investors, and healthcare providers. This analysis evaluates the current market landscape, competitive environment, regulatory factors, and provides future price projections based on market dynamics.
Market Overview
Global Market for Vitamin D Supplements
The global vitamin D supplement market has experienced exponential growth, driven by increasing awareness of vitamin D deficiency and its associated health risks, including osteoporosis, immune dysfunction, and autoimmune diseases [1]. The rising prevalence of vitamin D deficiency across developed and developing nations catalyzes market expansion. In 2022, the global vitamin D market was valued at approximately USD 900 million, with projections reaching USD 1.4 billion by 2030, growing at a CAGR of around 5% (2022-2030) [2].
Market Drivers
- Health Awareness: Growing public understanding of vitamin D's role in immune health, especially amidst the COVID-19 pandemic, stimulates consumer demand.
- Aging Population: Older demographics are increasingly supplementing to prevent osteoporosis and fracture risks.
- Preventive Healthcare Trends: Shift towards proactive health management promotes supplement use.
Regulatory Environment
Regulatory frameworks vary internationally; in the US, the FDA regulates dietary supplements but does not approve them before marketing. Conversely, in countries like India and parts of Southeast Asia, stricter regulations influence product pricing and market entry. Compliance with local standards significantly impacts manufacturing costs and, hence, selling prices [3].
Competitive Landscape
PUREVIT DUALFE PLUS CAPSULE faces competition from numerous vitamin D formulations—including monotherapy capsules, multivitamin combinations, and fortified foods. Key competitors typically include:
- Generic vitamin D brands: priced between USD 5-15 for comparable packages.
- Multivitamin complexes: often priced at USD 10-20, offering broader nutrient profiles.
- Premium formulations: incorporating additional bioactive compounds, priced above USD 20.
Brand recognition, formulation convenience, and scientific backing influence market positioning. As a dual-formulation product, PUREVIT DUALFE PLUS has unique value, potentially allowing for premium pricing compared to simple vitamin D supplements.
Pricing Analysis
Current Price Range
Based on market data, similar formulations typically retail in the following ranges:
- Standard vitamin D capsules: USD 0.10-0.50 per capsule.
- Premium dual-action capsules: USD 0.50-1.50 per capsule, depending on potency, brand strength, and formulation complexity [4].
Pricing Factors
- Manufacturing Costs: Ensure compliance with Good Manufacturing Practices (GMP) and active ingredient sourcing affect price.
- Regulatory Compliance: Licensing fees and certification costs raise the product’s cost base.
- Market Positioning: Premium branding and added ingredients justify higher prices.
- Distribution Channels: Direct-to-consumer online sales may reduce markups versus pharmacy or hospital sales.
- Regional Variations: Pricing differs significantly according to regional economic factors and healthcare policies.
Projected Price Trends
Given market growth and the increasing demand for fortified supplements, the following price progression is foreseeable:
| Year | Expected Average Retail Price (USD per capsule) | Rationale |
|---|---|---|
| 2023 | $0.80 - $1.00 | Initial stabilization post-launch, competitive adjustment |
| 2025 | $0.75 - $1.20 | Market expansion, inflation adjustments, strategic pricing sensitivity |
| 2030 | $0.70 - $1.50 | Price optimization driven by increased competition and product differentiation |
The pricing will likely stabilize toward the higher end if PUREVIT DUALFE PLUS capitalizes on its dual-action benefits and scientific validation.
Market Opportunities
The product's dual-faceted design could target:
- Immune health segments: capitalizing on immune-boosting claims.
- Osteoporosis prevention: marketing to aging populations.
- Preventive health markets: integrating into daily health routines.
Regional expansions, especially in emerging markets with rising health consciousness, offer growth prospects. Regulatory approvals and clinical evidence bolster the product’s premium positioning.
Challenges & Risks
- Price Sensitivity: Consumers in price-sensitive markets may prefer generic or single-nutrient products.
- Regulatory Hurdles: Stringent approval processes could delay market entry, impacting revenue forecasts.
- Market Saturation: Mature markets may experience slow price appreciation due to competitive pressure.
- Counterfeit Risks: Especially in high-growth regions, impacting brand integrity and pricing strategies.
Key Takeaways
- The global vitamin D market is expanding, driven by health awareness, aging demographics, and preventive healthcare trends.
- PUREVIT DUALFE PLUS’s dual-action formulation positions it favorably for premium pricing, typically ranging from USD 0.80 to USD 1.50 per capsule.
- Market entry and geographic expansion should consider regional regulatory, economic, and competitive factors.
- Strategic brand positioning, clinical validation, and regulatory compliance are crucial for maintaining premium pricing.
- Projected stabilization of prices indicates potential for sustained revenue growth, especially if marketing emphasizes unique benefits.
FAQs
1. How does PUREVIT DUALFE PLUS differ from standard vitamin D supplements?
PUREVIT DUALFE PLUS combines vitamin D with additional bioactive compounds, offering dual benefits like immune support and bone health, thus positioning itself as a premium, multifunctional supplement compared to single-vitamin formulations.
2. What regulatory considerations impact its pricing and market deployment?
Regulatory approval processes, localized certifications, and compliance with GMP standards influence manufacturing costs and initial pricing strategies, especially in diverse markets with varying standards.
3. How can market growth impact the long-term pricing of PUREVIT DUALFE PLUS?
Growth in demand and brand recognition allow for strategic price improvements, especially if product differentiation and clinical efficacy are emphasized, leading to higher margins and market share.
4. What competitive advantages does PUREVIT DUALFE PLUS have?
Its dual-action formulation offers unique benefits, potentially allowing premium pricing. Its positioning as a health-enhancing, scientifically validated product enhances consumer value perception.
5. Which regions present the most significant growth opportunities?
Emerging markets in Asia-Pacific, Latin America, and the Middle East show rapid growth driven by increasing health awareness, urbanization, and rising disposable incomes, making them attractive for expansion.
Sources:
- MarketWatch, "Vitamin D Market Size, Share & Industry Analysis," 2022.
- Fortune Business Insights, "Vitamin D Market Forecast," 2022.
- FDA Regulations on Dietary Supplements, 2022.
- IQVIA, "Global Dietary Supplement Pricing Analysis," 2022.
More… ↓
