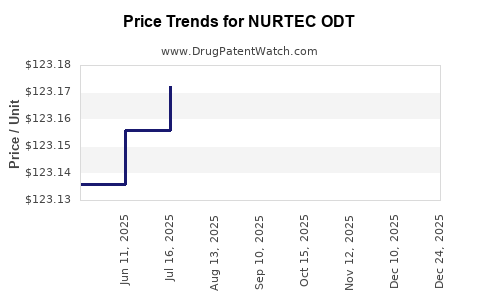
Last updated: December 1, 2025
Summary
NURTEC ODT (rimegepant), developed by Biohaven Pharmaceuticals, is an oral calcitonin gene-related peptide (CGRP) antagonist approved for acute and preventive treatment of migraine. Since its FDA approval in 2020, NURTEC ODT has gained significant market traction, driven by the increasing global migraine prevalence and shift towards targeted, oral therapies. This report analyzes the market landscape, key competitors, pricing strategies, and forecasts future price trends based on current data, regulatory factors, and market adoption.
What Is NURTEC ODT and Why Is It Marketed?
Product Overview
| Parameter |
Details |
| Generic Name |
Rimegepant |
| Brand Name |
NURTEC ODT |
| Formulation |
Orally disintegrating tablet (ODT) |
| Indication |
Acute treatment and preventive therapy for migraine |
| Approval Date |
October 2020 (FDA) |
| Manufacturers |
Biohaven Pharmaceuticals (original), subsequently marketed via Pfizer (post-merger)** |
Therapeutic Profile
- NURTEC ODT inhibits CGRP, a neuropeptide involved in migraine pathophysiology.
- The drug offers rapid onset, convenient oral administration, and dual indication (acute and preventive).
- It appeals to patients seeking alternatives to injectable CGRP monoclonal antibodies.
Market Landscape
Global Migraine Treatment Market
The migraine therapeutics market was valued at approximately $5.4 billion in 2021 and is projected to grow at a CAGR of 4.8% through 2028 [1]. The key drivers include:
| Factor |
Impact |
| Rising migraine prevalence |
Drives demand for effective treatments |
| Growing awareness |
Encourages diagnosis and treatment adherence |
| Innovation in CGRP therapies |
Expands treatment options |
Competitive Environment
| Product |
Type |
Mechanism |
Market Position |
Price (approx.) |
FDA Approval |
| NURTEC ODT (rimegepant) |
Oral CGRP antagonist |
Gepant |
First oral dual-use (acute & preventive) |
~$78 per tablet |
2020 |
| Ubrelvy (ubrogepant) |
Oral CGRP antagonist |
Gepant |
Acute treatment only |
~$54 per tablet |
2019 |
| Aimovig (erenumab), Ajovy (fremanezumab), Emgality (galcanezumab) |
Injectable CGRP monoclonal antibodies |
CGRP blockade |
Preventive |
$575–$675 per month |
2018–2019 |
Market Share Dynamics (2022–2023)
| Company |
Product |
Estimated U.S. Market Share (%) |
Notes |
| Biohaven/Pfizer |
NURTEC ODT |
~35 |
Leading oral CGRP agent for acute/preventive use |
| AbbVie |
Ubrelvy |
~20 |
Competitive acute therapy |
| Amgen/Novartis |
Aimovig, Ajovy, Emgality |
~25 |
Dominant preventive injections |
| Others |
- |
~20 |
Includes generics and emerging therapies |
Pricing Strategies and Factors Influencing NURTEC ODT Pricing
Current Price Structure
| Pricing Parameter |
Details |
| Average Wholesale Price (AWP) |
~$78 per tablet (U.S.) |
| Monthly Treatment Cost (e.g., 30 tablets) |
~$2,340 |
| Insurance & Copay |
Prices reduced via pharmacy benefit managers (PBMs) and copay assistance programs |
Pricing Drivers
- Market Positioning: Premium pricing justified by dual indication, rapid onset, and convenience.
- Reimbursement Policies: Payer negotiations influence net prices; high copay assistance programs mitigate patient costs.
- Cost of Development & R&D: Investment in clinical trials (~$100–$200 million) influences initial pricing.
- Competitive Landscape: The lower cost of some alternatives (e.g., generics) pressures premium pricing.
Reimbursement & Access Trends
| Payer Type |
Coverage Level |
Reimbursement Challenges |
| Medicare/Medicaid |
Favorable |
Restrictions depend on formulary placements |
| Commercial Insurance |
Widely covered |
High copays; step therapy policies |
Market Adoption and Growth Projections
Forecasts (2023–2028)
| Parameter |
Projection |
Source/Basis |
| Market Penetration (U.S.) |
40–50% of migraine patients |
Based on current adoption rates |
| Annual Revenue |
~$600 million by 2028 |
Considering market share growth, price adjustments |
| Number of Prescriptions (2022) |
~1.2 million |
IMS Health data |
| Compound Annual Growth Rate (CAGR) |
~12.5% |
Driven by increasing awareness and approvals |
Future Price Trends
| Factors |
Effect on Price |
| Market Competition |
Potential downward pressure due to generics and biosimilars |
| Regulatory Changes |
Price controls or negotiation policies (e.g., Medicare drug price negotiations) may limit pricing flexibility |
| Innovation and Extended Indications |
Additional approvals or combination therapies could support premium pricing |
| Projected Price Range (2024-2028) |
Estimated Price per Tablet |
| Conservative Scenario |
~$70–$75 |
| Aggressive Price Reduction Scenario |
~$60–$65 |
Comparative Analysis: NURTEC ODT vs. Alternatives
| Aspect |
NURTEC ODT |
Ubrelvy |
Injectables (Aimovig, Emgality, Ajovy) |
Other Gepants (e.g., Atogepant) |
| Formulation |
ODT |
Oral |
Injectable |
Oral |
| Indications |
Acute & preventive |
Acute |
Preventive |
Preventive |
| Pricing per Dose |
~$78 |
~$54 |
~$575/month (injectables) |
TBD |
| Onset of Action |
Rapid |
Rapid |
Varies |
Varies |
| Patient Preference |
Oral, convenient |
Oral |
Injectable, patient preference varies |
Oral |
Regulatory and Policy Considerations Impacting Pricing and Market
Regulatory Environment
- FDA Approvals: 2020 (NURTEC ODT), 2019 (Ubrelvy), 2018–2019 (injectables)
- EMA & Global Approvals: Pending, may influence international pricing
- Biosimilar and Generics: No biosimilars yet; potential future entrants may reduce prices
Healthcare Policies and Price Negotiation
- US Price Negotiation Initiatives: Medicare's increased authority to negotiate drug prices could cap prices.
- Capping Out-of-Pocket Costs: Legislative efforts aim to limit patient expenses, impacting revenue.
- Affordable Care Act (ACA): Expanded coverage may increase access but constrain pricing flexibility.
Key Takeaways
- NURTEC ODT holds a significant share of the oral migraine treatment market, with an innovative dual indication that enhances value.
- Pricing remains high (~$78 per tablet) but is tempered by insurance negotiations and patient assistance programs.
- Market growth is propelled by increasing migraine prevalence, heightened physician and patient acceptance of oral therapies, and improvements in reimbursement.
- Competitive pressures from generics, biosimilars, and emerging drugs could lead to price stabilization or reductions in the coming years.
- Regulatory and policy changes—especially US drug price negotiation initiatives—pose upside and downside risks to future pricing trajectories.
FAQs
1. What factors could lead to a decline in NURTEC ODT’s price?
Market entry of generics post-patent expiration, increased competition from new oral CGRP antagonists, and potential price negotiations by payers and government programs could pressure prices downward.
2. How does NURTEC ODT compare to injectable migraine preventives?
NURTEC ODT offers oral administration with both acute and preventive indications, whereas injectables like Aimovig focus solely on prevention, often at higher prices and with different patient preferences and adherence considerations.
3. What is the impact of insurance coverage on NURTEC ODT’s retail price?
Insurance negotiations, formulary placements, and copay assistance mitigate out-of-pocket costs, but net prices to payers influence the manufacturer’s revenue and future pricing strategies.
4. Will NURTEC ODT's price increase or decrease in the next five years?
Likely to stabilize or slightly decrease due to increased competition, patent expiry, and regulatory pressures, but premium positioning may sustain current levels if market conditions remain favorable.
5. What is the potential global market for NURTEC ODT?
While primarily approved in the US, regulatory expansion to Europe, Asia-Pacific, and emerging markets could increase revenue. Pricing policies in these regions will significantly influence market penetration and price.
References
[1] Grand View Research. "Migraine Market Size, Share & Trends Analysis Report," 2022.
[2] FDA. "NURTEC ODT (rimegepant) Approval Announcement," October 2020.
[3] IQVIA. "Global Pharmaceutical Market Data," 2022.
[4] Biohaven Pharmaceuticals. "NURTEC ODT Product Details," 2021.
[5] MarketWatch. "Migraine Therapeutics Market Analysis and Insights," 2023.