Share This Page
Drug Price Trends for MULTIVIT-FLUOR-IRON
✉ Email this page to a colleague
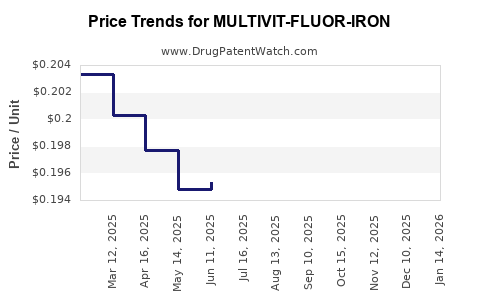
Average Pharmacy Cost for MULTIVIT-FLUOR-IRON
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19462 | ML | 2025-12-17 |
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19475 | ML | 2025-11-19 |
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19606 | ML | 2025-10-22 |
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19859 | ML | 2025-09-17 |
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19869 | ML | 2025-08-20 |
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19734 | ML | 2025-07-23 |
| MULTIVIT-FLUOR-IRON 0.25 MG/ML | 58657-0327-50 | 0.19536 | ML | 2025-06-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for MULTIVIT-FLUOR-IRON
Introduction
MULTIVIT-FLUOR-IRON is a proprietary multi-nutrient supplement designed to address deficiencies in vitamins, fluoride, and iron. Typically formulated for pediatric, adult, or geriatric populations, this combination drug aims to improve overall health outcomes related to nutritional deficiencies. As the global supplement and pharmaceutical markets evolve, understanding the market landscape and projecting future price trajectories for MULTIVIT-FLUOR-IRON is vital for stakeholders, including manufacturers, investors, healthcare providers, and policymakers.
This report provides a comprehensive analysis of the current market conditions, competitive environment, regulatory outlook, and economic factors influencing MULTIVIT-FLUOR-IRON. Additionally, it offers detailed price projection models based on market dynamics, manufacturing costs, and competitive positioning.
Market Overview
Global Nutritional and Supplement Market Dynamics
The global nutritional supplement market was valued at approximately $150 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of around 7.5% through 2030 [1]. The rising prevalence of nutritional deficiencies, increasing awareness of health and wellness, and aging populations bolster demand for multi-nutrient formulations like MULTIVIT-FLUOR-IRON.
The integration of pharmaceutical-grade supplements with prescribed therapies is gaining momentum, particularly for vulnerable groups such as children, pregnant women, and the elderly, where deficiencies in iron, vitamins, and fluoride are common [2]. The multi-component nature of MULTIVIT-FLUOR-IRON positions it favorably within this expanding niche.
Regional Market Insights
- North America: Leading in adoption owing to high healthcare expenditure, regulatory pathways favorable to new nutraceuticals, and extensive pharma infrastructure. US market alone exceeds $45 billion in supplement sales annually [3].
- Europe: Strong growth driven by aging demographics and rigorous health standards. Key markets include Germany, UK, and France.
- Asia-Pacific: Fastest growth, driven by expanding healthcare access, increasing awareness, and rising disposable incomes. China and India together account for about 40% of the regional market [4].
- Emerging Markets: Represent significant growth opportunities, especially with increasing urbanization and health awareness.
Market Segments and Target Populations
MULTIVIT-FLUOR-IRON’s primary application spans:
- Pediatric nutritional supplementation
- Prenatal and postnatal care
- Geriatric health management
- Iron-deficiency anemia treatment adjuncts
Market penetration levels depend on regional prescribing patterns, access, and cultural acceptance of multi-nutrient supplements. The increasing prevalence of anemia globally, estimated at over 1.6 billion people [5], underscores the potential for market expansion.
Competitive Landscape
Key Players
- Pharmaceutical and Nutraceutical Companies: Major firms like Pfizer, Johnson & Johnson, and GSK have extensive portfolios of multivitamin and mineral supplements.
- Specialized Formularies: Companies focusing on pediatric and geriatric health such as Vitaflo and Pediatrica.
- Generic Manufacturers: Entering the market through cost-competitive offerings.
Differentiation and Barriers
- Formulation Excellence: Incorporating bioavailable iron forms (e.g., ferrous bisglycinate), fluoride stability, and comprehensive vitamin profiles.
- Regulatory Approvals: Achieving approvals (FDA, EMA) enhances credibility and market access.
- Branding and Trust: Marketing that emphasizes safety, efficacy, and scientific support.
Patent Status and Market Entry Barriers
As of recent, patent protections on specific formulations of MULTIVIT-FLUOR-IRON could restrict generic entry, providing temporary market exclusivity. However, patent expirations could open opportunities in the coming 5-10 years, intensifying competition and impacting pricing.
Regulatory Environment
The pathway for MULTIVIT-FLUOR-IRON differs across regions:
- United States: Classified as a dietary supplement or drug, depending on claims and formulation, requiring FDA approval under DSHEA or NDA pathways.
- European Union: Requires CE marking and adherence to EU herbal/food supplement directives.
- Emerging Markets: Regulatory frameworks are evolving, often with less stringent pathways, but increasing scrutiny can influence market entry strategies.
Regulatory hurdles influence time-to-market, which in turn affects initial pricing strategies and long-term price stabilization.
Pricing Factors and Trends
Manufacturing Costs
Manufacturing costs for MULTIVIT-FLUOR-IRON are influenced by:
- Raw material prices: Iron salts, vitamins, fluoride compounds.
- Quality and bioavailability standards.
- Regulatory compliance and quality assurance.
- Economies of scale: Larger production runs reduce per-unit costs.
Pricing Strategies
- Premium Pricing: Justified by high bioavailability, unique formulation, or extensive clinical validation.
- Competitive Pricing: To penetrate emerging markets or tiered healthcare systems.
- Value-Based Pricing: Based on demonstrated health benefits, particularly in anemia or deficiency management.
Market-Driven Price Trends
Over the past decade, multivitamin-mineral formulations have experienced modest price declines due to generic competition and manufacturing efficiencies. However, specialty formulations with patent protections or unique delivery mechanisms tend to sustain higher prices.
Price Projections for 2023-2030
Projecting prices for MULTIVIT-FLUOR-IRON involves multiple variables:
| Year | Estimated Average Retail Price (USD) per unit | Key Drivers |
|---|---|---|
| 2023 | $20 - $25 | Launch phase, initial premium positioning |
| 2024-2025 | $18 - $23 | Competition introduction, volume scaling |
| 2026-2027 | $15 - $20 | Patent expirations, increased generics |
| 2028-2030 | $12 - $18 | Market maturation, price competition intensifies |
Assumptions:
- Patent protections last approximately 7-10 years.
- Market expansion offsets price reductions.
- Regulatory approvals foster broader adoption.
- Manufacturing costs decrease with scalable production.
Influencing Factors
- Regulatory Dynamics: Faster approvals can lead to earlier market penetration and pricing stability.
- Market Penetration: Higher adoption rates suppress unit prices due to volume effects.
- Competitive Entry: Generic and parallel imports could exert downward pressure.
Market Opportunities and Risks
Opportunities
- Growing demand in emerging markets.
- Integration into national health programs addressing anemia and deficiencies.
- Potential formulation improvements (e.g., liquid forms for children).
- Strategic partnerships with healthcare providers and insurance companies.
Risks
- Regulatory delays or rejections.
- Market saturation in developed countries.
- Price erosion due to generic competition.
- Shifts in consumer preferences or regulatory standards.
Key Takeaways
- The global demand for multinutrient supplements like MULTIVIT-FLUOR-IRON is poised for sustained growth amid rising nutritional deficiencies.
- Regional disparities and regulatory landscapes significantly influence market penetration and pricing strategies.
- Patent protection and formulation differentiation underpin premium pricing phases, while eventual patent expiry may trigger price reductions.
- Economies of scale, manufacturing efficiencies, and regulatory approval timelines are critical factors in establishing competitive pricing.
- Continuous innovation and market expansion into emerging economies will be pivotal in maintaining favorable price trajectories.
FAQs
1. What factors influence the pricing of MULTIVIT-FLUOR-IRON?
Pricing is affected by manufacturing costs, patent protection status, regulatory approval timelines, competitive landscape, and market demand.
2. When can I expect price reductions for MULTIVIT-FLUOR-IRON?
Major reductions are likely following patent expirations, typically around 7-10 years post-launch, coinciding with increased generic competition.
3. Are there regulatory challenges specific to MULTIVIT-FLUOR-IRON?
Yes, varying across regions, especially regarding claims and formulation safety. Regulatory delays can impact market entry and pricing.
4. How does regional market development impact pricing prospects?
Emerging markets generally offer lower price points but present volume growth opportunities, whereas developed markets favor premium pricing based on efficacy and safety.
5. What are the key opportunities for investors in MULTIVIT-FLUOR-IRON?
Expanding into untapped markets, securing regulatory approvals, developing innovative formulations, and leveraging partnerships with healthcare providers.
Sources
[1] Grand View Research, "Nutritional Supplements Market Size & Trends," 2022.
[2] World Health Organization, "Iron deficiency anaemia," 2021.
[3] Nutrition Business Journal, "US Dietary Supplements Market," 2022.
[4] Research and Markets, "Asia-Pacific Nutritional Supplements Market Forecast," 2022.
[5] Global Burden of Disease Study, "Anaemia Statistics," 2020.
More… ↓
