Share This Page
Drug Price Trends for MISOPROSTOL
✉ Email this page to a colleague
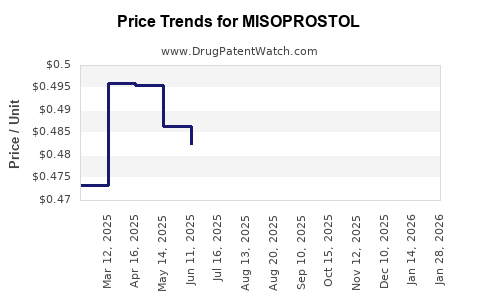
Average Pharmacy Cost for MISOPROSTOL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| MISOPROSTOL 100 MCG TABLET | 60687-0735-11 | 0.46191 | EACH | 2025-12-17 |
| MISOPROSTOL 100 MCG TABLET | 70954-0443-10 | 0.46191 | EACH | 2025-12-17 |
| MISOPROSTOL 100 MCG TABLET | 60687-0735-01 | 0.46191 | EACH | 2025-12-17 |
| MISOPROSTOL 100 MCG TABLET | 70954-0443-20 | 0.46191 | EACH | 2025-12-17 |
| MISOPROSTOL 100 MCG TABLET | 59762-5007-01 | 0.46191 | EACH | 2025-12-17 |
| MISOPROSTOL 200 MCG TABLET | 70954-0444-20 | 0.70116 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Misoprostol
Introduction
Misoprostol, a prostaglandin E1 analog, is a versatile pharmaceutical primarily used for the prevention and treatment of gastric ulcers, medical termination of pregnancy, and management of postpartum hemorrhage. Its low-cost production and high efficacy have cemented its role in reproductive health, especially in low- and middle-income countries (LMICs). As a critical drug with broad applications, understanding its market dynamics and price trajectories is vital for stakeholders, including manufacturers, healthcare providers, policymakers, and investors.
Market Overview
Global Market Size and Growth Dynamics
The global misoprostol market remains relatively resilient due to its essential applications. In 2022, the market was valued at approximately USD 150 million, with an expected compound annual growth rate (CAGR) of 4-6% through 2028. This growth is driven predominantly by increasing demand for reproductive health services, expanding access to medical abortion, and governmental initiatives to reduce maternal mortality [1].
Key Geographic Markets
- North America: Focused on medical abortion and postpartum hemorrhage management, with well-regulated markets and high acceptance.
- Europe: Growing use, particularly in countries with progressive reproductive health legislation.
- Asia-Pacific: The largest and fastest-growing segment, fueled by substantial demand in India, China, and Southeast Asia. The region's large population base and rising healthcare infrastructure undeline its market potential.
- Africa and Latin America: High dependency on low-cost, readily available medications; expanding market opportunities aligned with maternal health initiatives.
Regulatory Landscape
Misoprostol is classified as an essential medicine by the World Health Organization (WHO). The regulatory environment varies globally, with some countries requiring prescription control, while others permit over-the-counter sale for specific indications. Recent reforms in several jurisdictions aim to streamline approval processes, potentially expanding market access [2].
Market Drivers and Challenges
Drivers
- Increasing Demand for Medical Abortion: WHO estimates that over 56 million abortions occur annually, with a significant portion facilitated via medication, prominently featuring misoprostol [3].
- Maternal Mortality Reduction Efforts: Postpartum hemorrhage, a leading cause of maternal death, is effectively managed using misoprostol, prompting policy support and procurement programs.
- Low Competitive Barriers: Generic manufacturing routes and low production costs allow broad market entry and pricing flexibility.
- Global Reproductive Rights Initiatives: Numerous NGOs and governmental programs promote misoprostol access, particularly in underserved regions.
Challenges
- Regulatory Restrictions: Stringent regulations in high-income countries can restrict availability.
- Supply Chain Issues: Disruptions can impact affordability and inventory management.
- Potential for Misuse: Unauthorized distribution or unregulated sale may impinge on market stability and safety.
Pricing Landscape
Factors Influencing Pricing
Misoprostol pricing varies substantially based on formulation, packaging, regulatory status, and geographic region. Factors include:
- Manufacturing Cost: Generic producers benefit from economies of scale, maintaining low prices.
- Regulatory Approval Status: Brand-name drugs generally command higher prices than generics.
- Distribution and Supply Chain Efficiency: Well-established distribution networks reduce costs.
- Market Demand and Competition: Increased competition drives prices downward.
Current Price Benchmarks
- Global Price Range: In LMICs, a standard 200 mcg tablet costs approximately USD 0.10 to USD 0.30 per unit.
- High-Income Countries: Prices are higher, often USD 1-USD 2 per tablet, due to regulatory and patent considerations.
- Bulk Procurement: Large institutional purchasers, such as governments and NGOs, often negotiate prices below USD 0.10 per tablet.
Price Trends
Over the past decade, prices for common generic formulations have remained relatively stable, driven by bipartisan demand for affordable reproductive health solutions. However, recent policy shifts and increased manufacturing capacity are expected to stabilize or potentially further reduce retail prices.
Market Forecast and Price Projections (2023–2028)
Projected Market Growth
The market is forecasted to expand at a CAGR of 4-6%, supported by:
- Increased adoption in reproductive health programs
- Expansion in LMICs facilitated by global health initiatives
- Growing acceptance and legal reforms surrounding medical termination
Major players are investing in manufacturing capacity, with emerging markets expected to be primary drivers.
Price Projections
- Developing Countries: Prices are expected to decline modestly, averaging USD 0.08–USD 0.15 per tablet by 2028, driven by increased competition and procurement efficiencies.
- Developed Countries: Prices will remain relatively stable but may see slight increases for branded formulations due to regulatory costs. However, generic competition will likely keep prices within USD 1–USD 2 per tablet.
- Impact of Patent Expiry: Since most formulations are off-patent, generic manufacturers continue to dominate, sustaining low prices.
Strategic Considerations for Stakeholders
- Manufacturers: Focus on expanding production capacity, especially in emerging markets, to capitalize on demand and maintain low costs.
- Policymakers: Support regulatory environments that facilitate affordable access while ensuring safety.
- NGOs and Global Health Entities: Leverage bulk procurement and subsidization programs to further reduce costs.
- Investors: Monitor market drivers such as regulatory reforms and demand growth, which underpin pricing stability and expansion prospects.
Key Takeaways
- The misoprostol market exhibits steady growth, primarily propelled by expanding reproductive health needs globally.
- Market dynamics favor low-cost generics, maintaining affordability especially in LMICs.
- Price trajectories suggest stability with potential declines driven by increased competition and procurement efficiencies.
- Regulatory reforms and policy support significantly influence market supply and pricing.
- Strategic investments in manufacturing and distribution networks are essential for stakeholders to maximize value.
FAQs
-
What are the main applications of misoprostol?
Misoprostol is used for gastric ulcer prevention, medical termination of pregnancy, and management of postpartum hemorrhage. -
How does regulatory status affect misoprostol pricing?
Strict regulations in high-income countries often lead to higher prices due to supply limitations, while widespread generic availability in LMICs sustains low costs. -
What is the projected price trend for misoprostol in developing countries?
Prices are expected to decline slightly, averaging around USD 0.08–USD 0.15 per tablet by 2028, due to increased competition and procurement efficiencies. -
Are there patent barriers affecting misoprostol manufacturing?
Most misoprostol formulations are off-patent, enabling generic manufacturers to produce low-cost versions. -
How do global health initiatives impact misoprostol markets?
They promote procurement and distribution, often resulting in bulk purchasing agreements that lower prices and expand access.
References
[1] MarketWatch. (2022). Misoprostol Market Size, Share & Trends Analysis.
[2] World Health Organization. (2021). Essential Medicines List.
[3] WHO. (2020). Medical Management of Abortion.
More… ↓
