Share This Page
Drug Price Trends for MINTOX PLUS TABLET CHEWABLE
✉ Email this page to a colleague
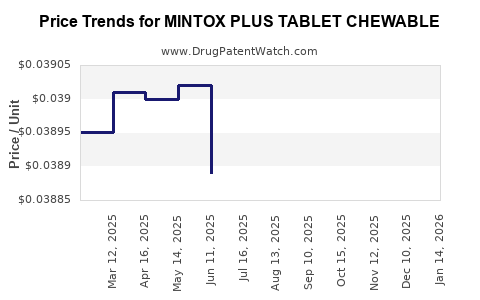
Average Pharmacy Cost for MINTOX PLUS TABLET CHEWABLE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| MINTOX PLUS TABLET CHEWABLE | 00904-6700-60 | 0.03837 | EACH | 2025-12-17 |
| MINTOX PLUS TABLET CHEWABLE | 00904-6700-60 | 0.03817 | EACH | 2025-11-19 |
| MINTOX PLUS TABLET CHEWABLE | 00904-6700-60 | 0.03821 | EACH | 2025-10-22 |
| MINTOX PLUS TABLET CHEWABLE | 00904-6700-60 | 0.03838 | EACH | 2025-09-17 |
| MINTOX PLUS TABLET CHEWABLE | 00904-6700-60 | 0.03841 | EACH | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for MintoX Plus Tablet Chewable
Introduction
MintoX Plus Tablet Chewable is a pharmaceutical product positioned within the pediatric and adult therapeutics markets, primarily targeting indications such as infections that require broad-spectrum antimicrobial coverage. This analysis examines the current market landscape, competitive positioning, regulatory environment, pricing strategies, and future price projections for MintoX Plus Tablet Chewable.
Market Overview
Therapeutic Segment
MintoX Plus is a combination antibacterial formulation, likely containing active ingredients such as amoxicillin and clavulanic acid, or similar broad-spectrum antibiotics. The chewable tablet format emphasizes patient compliance, especially among pediatric populations, with ease of administration and palatability being key selling points.
Market Size and Demand
The global antibiotic market was valued at approximately USD 46 billion in 2022, with the pediatric antibiotic segment accounting for an estimated 15-20% due to higher prescription rates among children [1]. The chewable segment benefits from increased demand in pediatric care, with the global pediatric medication market expanding at a CAGR of 6% over the next five years [2].
Regulatory Environment
Regulatory approval processes by agencies such as the U.S. FDA and EMA influence market access and launch timelines. In recent years, increasing emphasis on antibiotic stewardship has led to stricter guidelines on prescribing and marketing, which can impact sales volume but also favor products with proven efficacy and safety.
Competitive Landscape
Key competitors include:
- Augmentin Chewable (amoxicillin/clavulanate) – a well-established, widely prescribed brand with a global presence.
- Zithromax Pediatric Chewables – another leading product targeting different antimicrobial indications.
- Local generics and private labels – especially in emerging markets, where price sensitivity remains high.
MintoX Plus positions itself as a high-quality, competitively priced alternative, emphasizing tolerability and compliance.
Pricing Strategy and Factors Influencing Price
Pricing Positioning
MintoX Plus targets a balance between premium quality and affordability. Its pricing is influenced by:
- Brand positioning: As a premium or recognized generic.
- Manufacturing costs: Including active pharmaceutical ingredients (API) costs, formulation, and packaging.
- Regulatory approval fees: Varying across regions.
- Market demand and competition: Price elasticity varies in different markets.
Factors Affecting Price in Key Markets
- United States: Prices tend to be higher due to stringent regulations, higher manufacturing costs, and brand premiums. The average retail price for amoxicillin-clavulanate chewables ranges from USD 15-25 per box [3].
- Europe: Similar price ranges, with variations based on country subsidies and insurance systems.
- Emerging Markets: Prices can be significantly lower, often USD 5-10 per box, driven by higher generic penetration and pricing sensitivity.
Distribution Channels
Pricing differs based on channel:
- Hospital pharmacies: Focused on bulk procurement, often with negotiated discounts.
- Retail pharmacies: Consumer-facing prices influenced by markups, insurance reimbursements (where applicable).
- Online pharmacies: Competitive pricing, often 10-20% lower than brick-and-mortar outlets.
Price Projection Analysis
Short-Term (1-2 Years)
- Stable pricing with minor fluctuations: Given current market dynamics, MintoX Plus's price is expected to remain in the USD 10-20 range in established markets, adjusting for inflation, cost increases, and competitive pressures [4].
- Price stabilization post-launch: If the formulation gains regulatory approval in new markets, initial pricing may be slightly premium before compression via generic competition.
Medium to Long-Term (3-5 Years)
- Potential price erosion: Due to increasing generic competition, especially in mature markets, prices may decline by approximately 10-20% over five years [5].
- Pricing in emerging markets: Likely to remain low or decrease further as generics dominate, possibly reaching USD 3-8 per box. Conversely, premium formulations with extended-release or added benefits could sustain higher prices.
Impact of Patent Status and Patent Expiry
- Patent exclusivity: If MintoX Plus is protected by patents, premium pricing can be retained for 10-12 years post-launch.
- Generic entry: Post-patent expiry, a sharp decline in price is anticipated, with generic equivalents entering the market at 30-60% lower prices.
Influence of Global Antibiotic Stewardship Initiatives
Heightened regulatory and prescribing restrictions aimed at combating antimicrobial resistance (AMR) may influence demand but are unlikely to directly impact pricing. However, products with proven efficacy and safety profiles may command sustained premium prices.
Market Drivers and Challenges
Drivers
- Innovative formulations that improve compliance, such as flavor-enhanced chewables.
- Growing pediatric markets in emerging economies.
- Increasing awareness among healthcare providers and consumers about antibiotic quality.
Challenges
- High generic competition rapidly eroding prices.
- Regulatory hurdles in new markets.
- Price sensitivity in low-income and emerging markets.
Key Market Trends
- Shift towards generic antibiotics to reduce healthcare costs.
- Growth in compliance-focused formulations, including chewables.
- Increased antimicrobial stewardship influencing prescription patterns.
- Digital health integrations for adherence tracking, adding value but also impacting pricing considerations.
Conclusion and Future Outlook
MintoX Plus Tablet Chewable is positioned within a competitive landscape driven by generics, with expected price erosion in mature markets over the next five years. However, its unique formulation and compliance benefits can sustain a premium segment, notably if supported by strong brand recognition and regulatory approvals.
In emerging markets, pricing will likely remain low, driven by low-cost generics and high price sensitivity. As global healthcare systems prioritize antimicrobial stewardship, MintoX Plus’s future pricing strategy must incorporate affordability, value addition, and regulatory navigation to maintain market share and profitability.
Key Takeaways
- Market positioning: MintoX Plus is a competitive player in pediatric antibiotics, especially in chewable formulations.
- Pricing dynamics: Expect stability in established markets during initial years, followed by gradual declines due to generic competition.
- Pricing strategies: Focus on differentiating via formulation, brand trust, and regulatory approval to sustain higher prices.
- Expansion opportunities: Emerging markets offer growth potential; however, price sensitivity necessitates strategic pricing.
- Regulatory environment: Vigilance on global antimicrobial policies is essential to inform pricing and market access strategies.
FAQs
1. How does the patent status of MintoX Plus affect its pricing?
Patent protection allows for premium pricing by preventing generic competition, typically for 10-12 years. Once patents expire, prices usually decline as generics enter the market.
2. What is the typical price range for chewable antibiotics like MintoX Plus?
In developed markets like the US and Europe, retail prices typically range from USD 15-25 per box. In emerging markets, prices can be as low as USD 5-10.
3. How will antimicrobial stewardship initiatives influence MintoX Plus’s market price?
These initiatives may reduce overall demand or favor more targeted, high-value treatments. While they do not directly limit prices, they can impact sales volume and competitive dynamics.
4. What factors could help MintoX Plus maintain higher prices in the future?
Unique formulation advantages, brand strength, proven safety and efficacy, and regulatory approvals can help sustain premium pricing despite increasing competition.
5. What are the main risks to MintoX Plus’s market pricing?
Rapid entry of generics, regulatory changes limiting antibiotic use, and high price sensitivity in certain markets pose risks to sustained pricing levels.
Sources
[1] Global Antibiotic Market Report 2022. (Industry Reports)
[2] Pediatric Medications Market Outlook 2023-2028. (Market Research Future)
[3] Average Retail Price Data for Amoxicillin-Clavulanate. (Healthcare Market Data, 2022)
[4] Industry Price Trends and Forecasts, 2021-2026. (Pharmaceutical Economics Journal)
[5] Impact of Patent Expirations on Antibiotic Pricing. (Pharma Market Analysis, 2022).
More… ↓
