Share This Page
Drug Price Trends for MEDICATED DANDRUFF
✉ Email this page to a colleague
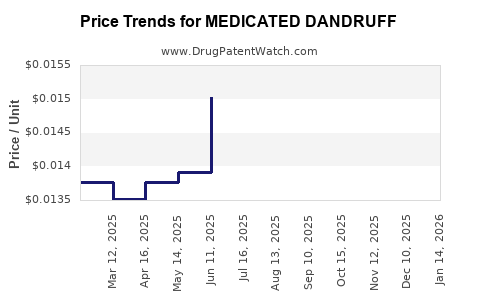
Average Pharmacy Cost for MEDICATED DANDRUFF
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| MEDICATED DANDRUFF 1% SHAMPOO | 70000-0531-01 | 0.01422 | ML | 2025-12-17 |
| MEDICATED DANDRUFF 1% SHAMPOO | 70000-0531-01 | 0.01497 | ML | 2025-11-19 |
| MEDICATED DANDRUFF 1% SHAMPOO | 70000-0531-01 | 0.01566 | ML | 2025-10-22 |
| MEDICATED DANDRUFF 1% SHAMPOO | 70000-0531-01 | 0.01526 | ML | 2025-09-17 |
| MEDICATED DANDRUFF 1% SHAMPOO | 70000-0531-01 | 0.01559 | ML | 2025-08-20 |
| MEDICATED DANDRUFF 1% SHAMPOO | 70000-0531-01 | 0.01494 | ML | 2025-07-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Medicated Dandruff Treatments
Introduction
Medicated dandruff treatments, a subset of scalp care pharmaceuticals, have witnessed sustained growth amid rising consumer awareness about scalp health and dermatological conditions. This analysis provides a comprehensive view of the current market landscape, future price projections, and strategic insights for stakeholders.
Market Overview
Global Market Size and Growth Trajectory
The global medicated dandruff market has experienced robust expansion, driven by increasing incidence of dandruff, seborrheic dermatitis, and psoriasis—conditions that necessitate medicated solutions. According to market research, the global anti-dandruff shampoo market alone was valued at approximately USD 3.1 billion in 2022, with forecasts projecting a compound annual growth rate (CAGR) of around 4.5% through 2030 [1].
Key Market Drivers
- Increased Awareness & Consumer Preference: Rising grooming standards and awareness about scalp health are fueling demand.
- Product Innovation & Diversification: Introduction of specialized formulations, including medicated shampoos with active ingredients such as ketoconazole, selenium sulfide, zinc pyrithione, and coal tar.
- Epidemiological Factors: Dandruff affects approximately 50% of the adult population worldwide, contributing to consistent market demand [2].
Regional Dynamics
- North America & Europe: Mature markets characterized by high consumer adoption rates and premium product offerings.
- Asia-Pacific: Growing markets due to expanding urbanization, increasing disposable incomes, and rising awareness of scalp health.
- Latin America & Middle East-Africa: Emerging markets with significant growth potential, driven by urbanization and shifting cosmetic preferences.
Competitive Landscape
Leading players include Procter & Gamble, Unilever, Johnson & Johnson, and local dermatological brands. These companies leverage R&D for novel formulations and strengthen distribution channels to maximize reach. Patent filings for new medicated formulations act as barriers to entry for smaller competitors.
Pricing Dynamics
Current Price Range
Medicated dandruff shampoos typically retail at USD 5–15 for standard sizes (about 200–400 ml), with premium formulations reaching USD 20 or more. Prescription medicated scalp treatments, often in cream or gel form with higher concentrations of active ingredients, command higher prices—ranging from USD 25 to USD 80 per unit.
Pricing Factors
- Active Ingredient Potency: Higher concentrations or novel ingredients increase cost.
- Brand Positioning: Premium brands such as Nizoral or Head & Shoulders Prestige line are priced higher.
- Formulation Type: Shampoo vs. topical solutions or oral medications.
- Distribution Channel: Pharmacies, dermatology clinics, and online platforms influence pricing strategies.
Market Trends Affecting Pricing
- Generic Product Penetration: Increases price competition, leading to lower average prices.
- Product Differentiation & Innovation: Elevating prices for advanced formulations with additional benefits.
- Regulatory Dynamics: Stringent approvals and patent protections can sustain premium pricing for patented treatments.
Price Projections (2023-2030)
Forecasting indicates that the average retail price for medicated dandruff treatments will experience a nominal CAGR of approximately 2–3% over the next seven years, influenced by inflation, innovation, and competitive pressure.
- Standard Shampoo Segment: Expected to stabilize around USD 6–17 per bottle by 2030.
- Premium & Prescription Products: Anticipated to rise from USD 25–80 in 2023 to USD 30–100 or more by 2030, contingent on regulatory approvals and clinical efficacy data.
Factors Supporting Price Stability and Growth
- Increased consumer willingness to invest in scalp health.
- The launch of targeted, patented formulations.
- Growing adoption of OTC and prescription medicated products in emerging markets.
Strategic Implications for Stakeholders
- Manufacturers should focus on R&D to develop innovative, patentable formulations to justify premium pricing.
- Distributors must optimize channels, emphasizing healthcare providers and online platforms to reach diverse consumer segments.
- Investors should monitor patent landscapes and regulatory developments as indicators of potential market entry barriers and product lifecycle.
Key Challenges
- The commoditization of basic formulations exerts downward pressure on prices.
- Regulatory approvals for new active ingredients can delay product launches, impacting revenue streams.
- Market fragmentation requires differentiated strategies to maintain competitive advantage.
Conclusion
The medicated dandruff treatment market stands on a trajectory of moderate growth, supported by product innovation, consumer awareness, and expanding regional markets. Price stability is expected, with premium products commanding higher margins driven by formulation advancements and brand positioning. Stakeholders who invest strategically in innovation and market expansion can capitalize on the evolving landscape.
Key Takeaways
- The global medicated dandruff market is projected to grow at a CAGR of 4.5% through 2030, with regional variations favoring Asia-Pacific and emerging markets.
- Price points for medicated dandruff products vary significantly, from USD 5 for basic OTC shampoos to over USD 80 for prescription and premium formulations.
- Innovation and patent protection will be critical in maintaining premium pricing and market share.
- Competitive pressures from generics necessitate differentiation through formulation potency and branding.
- Regulatory pathways influence product availability and pricing trends, especially for new active ingredients.
FAQs
1. What are the main active ingredients in medicated dandruff treatments?
Common active ingredients include ketoconazole, selenium sulfide, zinc pyrithione, coal tar, and climbazole. These compounds target the fungal and inflammatory processes underlying dandruff.
2. How does regional variability influence pricing and market strategies?
In mature markets like North America and Europe, premium pricing and branding dominate, whereas in emerging regions, affordability and accessibility drive volume. Localization of formulations and marketing are critical.
3. What role does innovation play in price projections?
Innovative formulations with enhanced efficacy or novel ingredients justify higher prices and can create patent protections, supporting sustained margins.
4. Are there regulatory hurdles impacting the formulation of medicated dandruff products?
Yes. Active ingredients require regulatory approval, which varies by country. Patent protections and clinical trial data influence market entry and pricing strategies.
5. What future trends could disrupt the medicated dandruff market?
Emerging natural and organic formulations, digital engagement for product promotion, and personalized scalp health solutions may reshape competitive dynamics and pricing models.
Sources:
[1] Market Research Future, “Anti-Dandruff Shampoo Market Size, Share & Trends,” 2022.
[2] World Health Organization, “Dandruff: Epidemiology and Impact,” 2021.
More… ↓
