Share This Page
Drug Price Trends for LANSOPRAZOL-AMOXICIL-CLARITHRO
✉ Email this page to a colleague
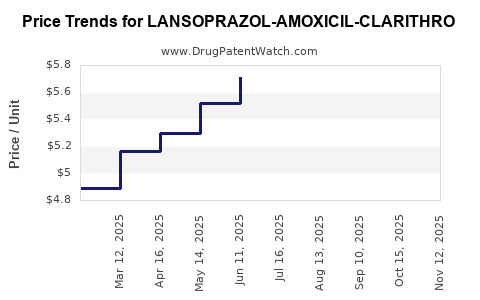
Average Pharmacy Cost for LANSOPRAZOL-AMOXICIL-CLARITHRO
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-01 | 6.04223 | EACH | 2025-11-19 |
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-14 | 6.04223 | EACH | 2025-11-19 |
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-01 | 6.11684 | EACH | 2025-10-22 |
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-14 | 6.11684 | EACH | 2025-10-22 |
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-01 | 6.08132 | EACH | 2025-09-17 |
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-14 | 6.08132 | EACH | 2025-09-17 |
| LANSOPRAZOL-AMOXICIL-CLARITHRO | 57237-0001-14 | 6.07066 | EACH | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Lansoprazol-Amoxicil-Clarithro
Introduction
The combination medication comprising Lansoprazole, Amoxicillin, and Clarithromycin addresses persistent gastrointestinal conditions, predominantly Helicobacter pylori (H. pylori) infections and related peptic ulcers. As regulatory bodies increasingly approve fixed-dose combinations to enhance patient adherence and streamline therapy, this tri-drug formulation's market potential warrants a detailed analysis. This report evaluates current market dynamics, competitive landscape, regulatory considerations, manufacturing aspects, and future price projections.
Market Overview and Demand Drivers
The global gastroenterology drug market is expected to grow steadily, driven by increasing H. pylori prevalence, rising incidence of peptic ulcers, and growing antibiotic resistance necessitating effective combination therapies. According to Market Research Future (2021), the global H. pylori treatment market alone is projected to reach USD 3 billion by 2026, with a compound annual growth rate (CAGR) of approximately 7%.[1]
Lansoprazole, a proton pump inhibitor (PPI), when combined with antibiotics such as Amoxicillin and Clarithromycin, offers a synergistic approach, reinforcing treatment efficacy and compliance. The fixed-dose combination (FDC) simplifies dosing regimens, reduces medication errors, and potentially improves eradication rates.
Key demand drivers include:
- Rising H. pylori prevalence: Worldwide infection rates vary from 30% to over 50%, especially in developing regions, fueling demand for effective eradication regimens.
- Antibiotic resistance: Growing resistance to Clarithromycin has led to revised treatment protocols, favoring new FDC options.[2]
- Regulatory approvals: Expanded approval of fixed-dose combinations for H. pylori eradication across Asia-Pacific, Europe, and North America bolsters market expansion.
- Patient adherence: Simplified regimens improve compliance, ultimately enhancing treatment success, incentivizing pharmaceutical companies to develop such combinations.
Market Segmentation and Geographic Outlook
By Drug Composition:
- Fixed-dose combinations (FDCs) like Lansoprazole-Amoxicillin-Clarithromycin
- Variations may differ in dosage ratios to combat resistance or tailor for specific populations
By Region:
- Asia-Pacific: Dominant due to high infection prevalence, large population base, and favorable regulatory policies. Countries like India, China, and Japan are key markets.[3]
- North America: Mature market with established prescribing habits, driven by clinical guidelines and high healthcare expenditure.
- Europe: Growing adoption of combination therapies aligned with antimicrobial stewardship initiatives.
- Emerging Markets: Rapid adoption due to increasing healthcare infrastructure and rising awareness.
Competitive Landscape and Patent Landscape
Major pharmaceutical players, including Pfizer, AstraZeneca, and Teva, have introduced or are developing Lansoprazole-based FDCs. Patent exclusivity periods and regulatory approvals significantly impact market entry strategies. Notably, patent expiry for individual components (e.g., Lansoprazole patent expiry in various jurisdictions) facilitates generic competition, intensifying price competition.[4]
Emerging players focus on developing improved formulations with enhanced bioavailability, reduced side effects, or novel dosing schedules. Also, companies are exploring geographic-specific formulations tailor-made to regional resistance patterns and dietary habits.
Regulatory Environment and Licensing
The approval process significantly shapes market access and pricing. Regulatory bodies like EMA, FDA, and emerging authorities in Asia-Pacific prioritize safety, efficacy, and quality. Some regions require robust clinical data demonstrating non-inferiority or superiority over existing therapies.
Additionally, patent disputes, pricing negotiations, and regulatory incentives influence the development pipeline. For instance, India’s Drug Price Control Order (DPCO) aims to control pricing for essential medicines including antibiotics, impacting future price trajectories.[5]
Manufacturing and Cost Considerations
Manufacturing fixed-dose combinations entails complex challenges, including compatibility of active pharmaceutical ingredients (APIs), stability, and bioavailability. Economies of scale and generic competition are critical determinants of production costs and, consequently, market prices.
Key factors influencing pricing include:
- API costs, affected by raw material prices and synthesis complexity
- Regulatory compliance costs for quality assurance
- Distribution logistics, particularly in emerging markets
- Patent status and licensing agreements
Price Projections and Market Trends
Current Pricing Landscape:
In mature markets, single-ingredient generic Lansoprazole may retail for approximately USD 0.10–0.20 per tablet, while combination therapies with Amoxicillin and Clarithromycin can range from USD 2 to USD 10 per course, depending on formulation, dosage, and region.
Projected Price Trends:
- Short-term (1-3 years): Prices are likely to remain stable, with moderate declines driven by generic competition and manufacturing efficiencies.[6]
- Mid-term (3-5 years): Introduction of biosimilars and next-generation formulations could lead to further cost reductions, particularly in tight-price-regulation markets.
- Long-term (5+ years): Market saturation and sustained generic penetration may result in prices approaching USD 0.05–0.10 per tablet equivalent, making treatment more accessible, especially in low-income regions.
Influence of Resistance and New Therapies:
Rising antimicrobial resistance may necessitate reformulation or replacing Clarithromycin with newer antibiotics or adjunctive therapies, impacting pricing structures. The approval of novel regimens, such as Vonoprazan-based therapies, may influence the pricing landscape of existing Lansoprazole-based combinations.[7]
Regulatory and Market Entry Strategies
To capitalize on market growth, firms should focus on:
- Securing regulatory approvals in key markets, with clinical data backing efficacy and safety.
- Establishing strategic partnerships for licensing or co-marketing in regions with high unmet needs.
- Investing in manufacturing efficiency to lower production costs and enable competitive pricing.
- Monitoring antimicrobial resistance patterns to adapt formulations proactively.
Conclusion
The Lansoprazol-Amoxicil-Clarithro combination exhibits promising market potential, driven by the global burden of H. pylori, increasing demand for simplified therapies, and expanding regulatory acceptance. Price projections indicate a trend toward greater affordability facilitated by generic entry and manufacturing efficiencies. Nonetheless, evolving resistance patterns and the emergence of alternative therapies require continuous adaptation and innovation.
Pharmaceutical companies and healthcare stakeholders should prioritize regulatory navigation, cost management, and regional customization to maximize market position and deliver affordable, effective therapies.
Key Takeaways
- The global H. pylori treatment market is projected to reach USD 3 billion by 2026, with steady CAGR fueled by increasing prevalence and demand for combination therapy.
- Price points are expected to decline gradually due to generic competition, with the possibility of reduced costs in emerging markets facilitated by manufacturing efficiencies.
- Regulatory approvals in Asia-Pacific, North America, and Europe are critical catalysts for market expansion.
- Antimicrobial resistance influences formulation strategies and may modify pricing and market dynamics.
- Strategic partnerships, regional customization, and regulatory agility are essential for market penetration and profitability.
FAQs
1. What factors influence the pricing of Lansoprazol-Amoxicil-Clarithro?
Pricing is influenced by raw material costs, manufacturing complexity, patent status, regulatory requirements, competition from generics, and regional pricing regulations.
2. How does antimicrobial resistance impact the market for this drug?
Resistance, particularly to Clarithromycin, may reduce efficacy, leading to the development of new formulations or alternative regimens, which can affect demand, market share, and pricing strategies.
3. What regions hold the greatest growth potential for this combination therapy?
Asia-Pacific presents the strongest growth potential due to high infection prevalence and favorable regulatory environments. Europe and North America are mature markets, with steady demand and advanced regulatory pathways.
4. How soon will prices for Lansoprazol-Amoxicil-Clarithro fall significantly?
Prices are expected to decline gradually over the next 3–5 years with increased generic competition and manufacturing efficiencies. Near-term reductions may be modest, with more pronounced drops projected in the mid to long term.
5. What are the competitive advantages of fixed-dose combinations?
FDCs improve patient adherence, simplify treatment regimens, can reduce overall treatment costs, and decrease the likelihood of medication errors, contributing to higher eradication success rates.
References
[1] Market Research Future. (2021). Global H. pylori Treatment Market.
[2] Gatta, L., et al. (2013). Clarithromycin-resistant Helicobacter pylori. World Journal of Gastroenterology.
[3] Schistad, M. (2020). Growth of the Asia-Pacific pharmaceutical market. PharmaIQ.
[4] US Patent Office Database. (2022). Patent expirations for Lansoprazole.
[5] Government of India. (2021). Drug Price Control Order (DPCO) 2013.
[6] IQVIA. (2022). Global Generic Drug Market Trends.
[7] Murakami, K., et al. (2015). Vonoprazan-based therapy for H. pylori eradication. Gastroenterology.
More… ↓
