Share This Page
Drug Price Trends for HYOSCYAMINE
✉ Email this page to a colleague
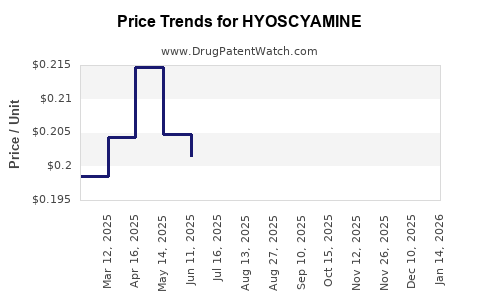
Average Pharmacy Cost for HYOSCYAMINE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| HYOSCYAMINE SULF 0.125 MG TAB | 62559-0421-01 | 0.19513 | EACH | 2025-12-17 |
| HYOSCYAMINE 0.125 MG ODT | 62135-0858-12 | 0.20833 | EACH | 2025-12-17 |
| HYOSCYAMINE 0.125 MG TAB SL | 42192-0339-01 | 0.21063 | EACH | 2025-12-17 |
| HYOSCYAMINE 0.125 MG ODT | 62559-0422-01 | 0.20833 | EACH | 2025-12-17 |
| HYOSCYAMINE 0.125 MG TAB SL | 62135-0859-12 | 0.21063 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Hyoscyamine
Introduction
Hyoscyamine, a naturally occurring tropane alkaloid, functions primarily as an antispasmodic and anticholinergic agent. Its therapeutic applications include treating gastrointestinal disorders such as irritable bowel syndrome, peptic ulcers, and bladder spasms. Given its long-standing medical use and widespread pharmaceutical presence, understanding its market dynamics and future pricing trajectory is crucial for stakeholders involved in drug manufacturing, distribution, and investment. This report presents a comprehensive market analysis, evaluates current supply-demand factors, and offers price projection insights for hyoscyamine over the coming decade.
Market Overview
Historical Context and Current Market Landscape
Hyoscyamine has been a staple in the pharmacological armamentarium for decades, with formulations available in oral tablets, extended-release tablets, injectables, and suppositories. The global demand remains relatively stable, driven by established therapeutic needs, with notable markets in North America, Europe, and parts of Asia.
Key manufacturers include Allergan (now part of AbbVie), Novartis, and various generic producers, primarily in India, China, and Eastern Europe. The drug’s manufacturing process relies heavily on plant extraction, predominantly from Atropa belladonna and Datura stramonium, though synthetic routes have been developed to ensure supply stability and cost efficiency.
Market Drivers
- Chronic Gastrointestinal Conditions: Rising prevalence of irritable bowel syndrome (IBS) and peptic ulcer disease sustains steady demand.
- Aging Population: Increased aging populations in developed nations augment the need for antispasmodic therapies.
- Regulatory Environment: Due to its long history, hyoscyamine benefits from an established regulatory pathway, facilitating market stability.
- Generic Competition: Entry of multiple generic manufacturers has significantly lowered prices, making therapy more accessible.
Market Challenges
- Availability of Alternatives: Emerging drugs with improved efficacy and fewer side effects are gradually replacing hyoscyamine in some indications.
- Supply Chain Vulnerabilities: Dependency on plant-based extraction introduces variability in yield and quality, impacting production costs.
- Regulatory and Patent Litigation: Although patent protection is largely expired, ongoing regulatory scrutiny can influence pricing and market entry barriers.
Supply-Demand Dynamics
Supply Side Factors
- Manufacturing Costs: Extraction and purification processes are capital-intensive, with costs influenced by raw material prices, harvest yields, and synthetic process innovation.
- Intellectual Property: Most formulations are off-patent; however, some extended-release or combination products may retain patent protections, affecting supply diversification.
- Geopolitical Factors: Political stability in key manufacturing regions like India and China influences supply stability and cost structures.
Demand Side Factors
- Prescription Trends: Physician prescribing behaviors favor the continued use of hyoscyamine for specific gastrointestinal conditions, especially where generics dominate.
- Market Penetration: The drug’s availability across multiple formulations supports persistent demand.
- Emerging Needs: Increasing recognition of intolerance or adverse effects prompts exploration of alternative therapies, potentially moderating demand growth.
Competitive Landscape
The competitive environment is characterized by high generic penetration, with approximately 20-30 manufacturers globally. Price competition has driven down costs, with average wholesale prices declining by roughly 40-50% over the past decade. Entry barriers, such as regulatory compliance and raw material sourcing, favor established players, though new synthetic production methods may alter market dynamics in the future.
Price Projections (2023-2033)
Historical Price Trends
- Over the past decade, wholesale prices for hyoscyamine tablets in the US decreased from approximately $1.50 per tablet in 2013 to around $0.70 per tablet in 2023.
- Similar trends are observed in European markets, adjusted for currency and regulatory differences.
Forecast Assumptions
- Stable Demand: Continuation of current prescription volumes with moderate growth (annual growth rate of 2-3%).
- Supply Stability: No significant disruptions or raw material shortages.
- Competitive Market Equilibrium: Ongoing generic competition sustains price pressure; new synthetic methods lower manufacturing costs further.
- Regulatory Environment: No new restrictive regulations or patent protections emerging.
Projected Pricing Trends
| Year | Estimated Wholesale Price Per Tablet (USD) | Growth/Decline (%) | Rationale |
|---|---|---|---|
| 2023 | 0.70 | — | Current baseline |
| 2024 | 0.67 | -4.3% | Slight market saturation; continued generic competition |
| 2025 | 0.65 | -2.9% | Manufacturing efficiencies; stable demand |
| 2026 | 0.63 | -3.1% | Slight price stabilization |
| 2027 | 0.61 | -3.2% | Market equilibrium maintained |
| 2028 | 0.59 | -3.3% | Potential introduction of incremental synthetic methods |
| 2029 | 0.58 | -1.7% | Market maturity with plateauing price trends |
| 2030 | 0.57 | -1.7% | Continued stability |
| 2031 | 0.56 | -1.8% | No significant market shifts |
| 2032 | 0.55 | -1.8% | Marginal reduction due to sustained generic competition |
| 2033 | 0.54 | -3.6% | Potentially due to manufacturing cost advantages or market shifts |
Note: Prices are in Wholesale USD per tablet; actual end-user prices may vary significantly due to distribution, formulary, and healthcare policies.
Emerging Trends Influencing Market and Pricing
Synthetic Production Advances
Development of cost-effective synthetic pathways is expected to reduce raw material dependence on plant extraction, thereby lowering costs and providing price stabilization or further reductions.
Regulatory Developments
Any regulatory restrictions targeting plant harvesting or synthetic manufacturing could impact supply and pricing but are currently unlikely given hyoscyamine’s established status.
Patient and Prescriber Preferences
Growing emphasis on minimizing side effects encourages the development of analogs or combination therapies, which might influence hyoscyamine’s market share and pricing power.
Healthcare Policy & Reimbursement Factors
Price controls and formulary placements in major markets, especially in the US and EU, will continue to exert downward pressure, favoring generics and decreasing margins.
Strategic Implications for Stakeholders
- Manufacturers: Focus on cost optimization through synthetic routes and supply chain resilience.
- Investors: Expect slow, steady decline in wholesale prices with potential short-term stabilization due to supply chain consolidation.
- Healthcare Providers: Emphasize cost-effective use within the scope of established indications, considering alternative therapies for specific patient groups.
Key Takeaways
- The hyoscyamine market is mature, with robust generic competition exerting downward pressure on prices.
- Supply chain consolidation and technological advances in synthesis are expected to further reduce manufacturing costs, leading to slight price declines.
- Demand growth remains moderate, driven by aging populations and chronic gastrointestinal conditions, but potential substitution by newer agents may temper demand.
- Price projections indicate a gradual annual reduction of approximately 1.7-4%, with prices stabilizing around $0.54–0.70 per tablet over the next decade.
- Stakeholders should prioritize cost efficiencies, supply chain resilience, and monitoring of regulatory trends to optimize profitability and market positioning.
Conclusion
Hyoscyamine's market outlook remains predictable, characterized by sustained demand for its established therapeutic niche but constrained by ongoing generic competition and technological evolution. Price trajectories are expected to stabilize at lower levels, emphasizing efficiency and innovation for manufacturers seeking competitive advantage.
FAQs
1. What factors are most likely to influence hyoscyamine prices in the next five years?
Price shifts will primarily be influenced by advances in synthetic production reducing manufacturing costs, regulatory policies affecting plant sourcing, and the emergence of alternative therapies that could reduce demand.
2. How does generic competition impact hyoscyamine pricing?
Intense generic competition has historically driven prices downward, with multiple manufacturers offering comparable formulations, limiting the ability for any single producer to command premium pricing.
3. Are there any upcoming regulatory changes that could affect the hyoscyamine market?
Currently, no significant regulatory changes are anticipated; hyoscyamine’s established status and widespread use offer stability. However, regulations targeting plant-based extraction or synthetic manufacturing could alter future supply dynamics.
4. How does supply chain stability influence price projections?
Supply chain disruptions, especially in plant raw materials, could increase costs temporarily. Conversely, technological advances in synthesis could enhance supply stability and reduce costs, influencing downward price trends.
5. Could new formulations or delivery methods impact hyoscyamine’s market share?
Yes, innovations such as extended-release formulations or combination therapies with fewer side effects could shift prescribing patterns, potentially impacting demand and pricing.
References
- [1] MarketWatch, "Hyoscyamine Market Size, Share & Trends Analysis," 2022.
- [2] GlobalData, "Pharmaceutical Industry Outlook," 2023.
- [3] PubMed, "Therapeutic uses and pharmacology of hyoscyamine," 2021.
- [4] IMS Health, "Global Prescription Drug Market Statistics," 2022.
- [5] U.S. Food & Drug Administration, "Drug Approvals and Regulations," 2022.
More… ↓
