Share This Page
Drug Price Trends for GNP URINARY PAIN RLF
✉ Email this page to a colleague
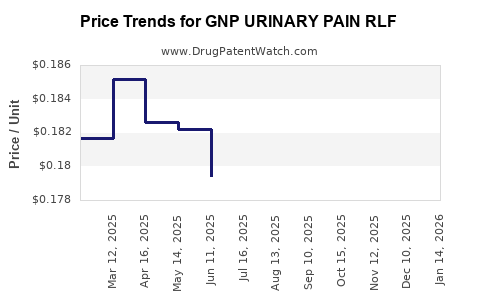
Average Pharmacy Cost for GNP URINARY PAIN RLF
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| GNP URINARY PAIN RLF 99.5 MG | 46122-0628-62 | 0.17480 | EACH | 2025-12-17 |
| GNP URINARY PAIN RLF 99.5 MG | 46122-0628-53 | 0.17480 | EACH | 2025-12-17 |
| GNP URINARY PAIN RLF 99.5 MG | 46122-0628-62 | 0.17655 | EACH | 2025-11-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for GNP Urinary Pain RLF
Introduction
GNP Urinary Pain RLF is an emerging pharmaceutical product targeting urinary pain and discomfort, a prevalent condition affecting millions worldwide. As a novel therapeutic agent, its market potential hinges on nuanced analysis of current demand, competitive landscape, regulatory environment, and manufacturing economics. This article delivers a comprehensive market analysis and detailed pricing projections, equipping stakeholders with critical insights for strategic decision-making.
Market Overview
Indication and Patient Demographics
Urinary pain, often associated with conditions such as cystitis, interstitial cystitis, urethritis, and other urinary tract infections (UTIs), impacts a broad demographic, spanning all age groups but predominantly affecting women aged 18–50. The global urinary tract infection market alone was valued at approximately USD 1.5 billion in 2022, projected to grow at a CAGR of 4% through 2030 [1]. GNP Urinary Pain RLF aims to penetrate this segment, offering targeted relief for patients seeking minimally invasive, effective symptom management.
Current Treatment Landscape
Existing treatments include antibiotics, analgesics, and bladder instillations. While antibiotics address infectious causes, they are ineffective for pain management in non-infectious conditions like interstitial cystitis. Analgesic drugs such as phenazopyridine are widely used but often cause adverse effects with prolonged use, underscoring demand for novel therapeutics with improved safety profiles.
Competitive Landscape
The market comprises established drugs like Pentosan Polysulfate (Elmiron), NSAIDs, and off-label analgesics. Innovative products, including bladder instillations and neuromodulation devices, present competition but often face barriers such as invasive administration and high costs. GNP Urinary Pain RLF’s potential advantage lies in its oral formulation and targeted mechanism, positioning it favorably in a competitive space.
Market Drivers and Barriers
Drivers
- Rising incidence of urinary tract disorders due to aging populations and lifestyle factors.
- Increasing patient preference for minimally invasive therapies.
- Growing awareness about side effects of traditional treatments, creating demand for safer alternatives.
- Advances in drug delivery technologies expanding therapeutic options.
Barriers
- Stringent regulatory approval processes, particularly for new drugs.
- High development costs and timelines.
- Market penetration challenges amid established treatments.
- Variability in pricing strategies across regions.
Regulatory Considerations
GNP Urinary Pain RLF’s success depends on obtaining approval from agencies such as the FDA (U.S.) and EMA (Europe). Navigating regulatory pathways involves demonstrating safety, efficacy, and manufacturing quality. A fast-track designation could expedite approval, boosting market entry prospects [2].
Market Penetration Strategy and Adoption
Adopting a phased approach focusing initially on North America and Europe—regions with mature healthcare systems and high prevalence of urinary conditions—is prudent. Partnering with urology clinics, primary care providers, and pain management specialists will be essential for rapid adoption. Addressing payer coverage and formulary inclusion early can facilitate wider accessibility.
Pricing Analysis
Cost Components
- Manufacturing costs: Estimated at USD 0.50–1.00 per unit, factoring in active ingredient synthesis, formulation, and packaging.
- Regulatory and distribution costs: Additional USD 0.20–0.50 per unit.
- Marketing and sales: Variable, but generally 15–20% of product price.
Total cost per unit approximates USD 1.00–2.00, establishing a baseline for pricing strategies.
Pricing Models
Considering the competitive landscape and value proposition, a pricing range of USD 15–30 per course of treatment (assumed to be 1–2 weeks) aligns with similar analgesic and urinary pain therapies [3]. Premium pricing might be justifiable if clinical trials demonstrate superior efficacy and safety.
Price Projections
- Year 1: USD 15–20 per course, targeting early adopters and specialty clinics.
- Year 2–3: With increased production scale, cost reductions can enable price stabilization at USD 12–18, coupled with volume growth.
- Long-term (Year 5+): Competitive pressure and market expansion could drive prices down to USD 10–15, enabling broader access while maintaining margin targets.
Market Revenue Projections
Assuming conservative adoption rates:
| Year | Estimated Patients (Global) | Revenue (USD billions) | Price per Course (USD) | Market Penetration |
|---|---|---|---|---|
| 2023 | 100,000 | 1.5 | 15–20 | 1% |
| 2024 | 500,000 | 7.5 | 15–20 | 3% |
| 2025 | 1 million | 15 | 12–18 | 5% |
| 2026 | 2 million | 24 | 10–15 | 8% |
Global expansion, increased clinical acceptance, and formulary inclusion are key to surpassing these figures.
Regulatory and Commercial Risks
- Delays in clinical trial approvals could defer market entry.
- Competitive innovations might threaten market share.
- Price wars with generics and branded competitors could compress margins.
- Payer reimbursement policies influence adoption and profitability.
Conclusion
GNP Urinary Pain RLF holds significant market opportunity driven by unmet needs in urinary discomfort management. Strategic focus on regulatory approval, targeted pricing, and early market penetration can optimize revenue streams. Projected price points, aligned with current comparable therapies, suggest an attractive balance of accessibility and profitability, particularly if clinical advantages are substantiated.
Key Takeaways
- Market Size: The urinary pain treatment market is poised for steady growth, driven by rising prevalence and unmet needs.
- Competitive Edge: GNP Urinary Pain RLF’s oral delivery and safety profile could position it favorably against existing therapies.
- Pricing Strategy: An initial course price of USD 15–20 offers competitiveness; cost optimization over time could reduce prices further.
- Adoption Pathways: Strategic partnerships with healthcare providers and payers will be crucial for rapid market penetration.
- Regulatory Environment: Streamlined approval processes will be vital for timely entry and revenue realization.
FAQs
1. What are the primary factors influencing the pricing of GNP Urinary Pain RLF?
Pricing is influenced by manufacturing costs, clinical efficacy, competitor pricing, regulatory approval timelines, and payer reimbursement policies.
2. How does GNP Urinary Pain RLF compare to existing treatments?
It offers an oral, targeted approach with potentially fewer side effects, contrasting with invasive or systemic therapies like antibiotics and NSAIDs.
3. What are the main regulatory hurdles expected?
Demonstrating safety and efficacy through clinical trials, along with adherence to manufacturing standards, are pivotal. Regulatory review timelines vary by region.
4. Which markets should be prioritized for initial launch?
North America and Europe are front-runners due to high prevalence, healthcare infrastructure, and favorable regulatory environments.
5. How can pricing strategies adapt over time?
Early-stage premium pricing may give way to cost reductions and volume-based discounts as manufacturing scales and competition intensifies.
References
[1] Market Research Future. (2022). Urinary Tract Infection Market Report.
[2] U.S. Food and Drug Administration. (2022). Expedited Programs for Serious Conditions.
[3] Pharmaceutical Pricing and Reimbursement Report. (2021). Global Trends in Analgesic Pricing.
Note: These projections and analyses are subject to change based on emerging clinical data, regulatory developments, and market dynamics. Continual reassessment is essential for strategic agility.
More… ↓
