Share This Page
Drug Price Trends for GNP LANSOPRAZOLE DR
✉ Email this page to a colleague
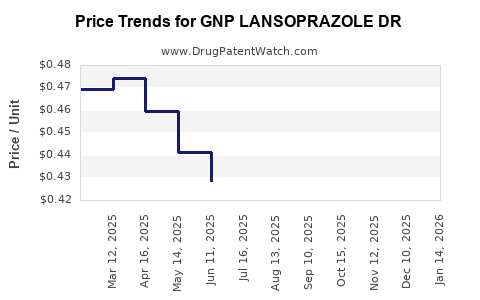
Average Pharmacy Cost for GNP LANSOPRAZOLE DR
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-04 | 0.42362 | EACH | 2025-12-17 |
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-74 | 0.42362 | EACH | 2025-12-17 |
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-04 | 0.43495 | EACH | 2025-11-19 |
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-74 | 0.43495 | EACH | 2025-11-19 |
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-04 | 0.44583 | EACH | 2025-10-22 |
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-74 | 0.44583 | EACH | 2025-10-22 |
| GNP LANSOPRAZOLE DR 15 MG CAP | 46122-0744-04 | 0.45025 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for GNP Lansoprazole DR
Introduction
GNP Lansoprazole DR (delayed-release) is a proton pump inhibitor (PPI) primarily used for treating conditions like gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger-Ellison syndrome. As a generic formulation, its market landscape is influenced by patent expiration, competitive dynamics, manufacturing capacity, and evolving prescribing trends. This comprehensive analysis explores the current market environment, competitive positioning, key growth drivers, and future price projections for GNP Lansoprazole DR over the coming years.
Market Overview
Global Demand and Use Cases
The global demand for PPIs, including Lansoprazole, continues to grow driven by increasing prevalence of acid-related gastrointestinal disorders. According to MarketsandMarkets, the global PPI market was valued at approximately USD 11.4 billion in 2020 and is projected to reach USD 16.9 billion by 2026, growing at a CAGR of around 6.8% (1). Lansoprazole, being available as a generic, accounts for a substantial share in prescription volumes, particularly in North America, Europe, and parts of Asia.
Patents and Market Entry
Lansoprazole originally held a primary patent which expired in the early 2010s, paving the way for numerous generics. GNP's branding as Lansoprazole DR positions it within this commoditized segment but with a focus on delayed-release formulations that may offer improved gastric tolerability and patient compliance, thus maintaining its relevance.
Regulatory and Reimbursement Dynamics
Regulatory pathways in key markets—including the United States (FDA), European Union (EMA), and Asia-Pacific (PMDA, NMPA)—affect market penetration. Reimbursement policies favor generic accessibility to control healthcare costs, which generally supports competitive pricing but also exerts downward pressure on prices.
Competitive Landscape
GNP Lansoprazole DR competes with several generic manufacturers, including Teva, Sandoz, Mylan, and others. The competition is characterized by:
- Pricing strategies: Aggressive price reductions to gain market share.
- Formulation differences: Variants like dispersible or dispersible tablets, and formulations with enhanced bioavailability.
- Market penetration: Established presence in urban and rural healthcare settings.
Additionally, branded PPIs like Nexium andPrevacid, although more expensive, still maintain niches in gastroenterology for specific indications, but they have limited impact on the generic Lansoprazole segment.
Market Drivers
Increasing Prevalence of Acid-Related Disorders
Rising cases of GERD, peptic ulcer disease, and Barrett's esophagus globally sustain demand. Lifestyle factors such as obesity and dietary habits contribute to this trend, especially in developed and emerging markets.
Growing Aging Population
Older adults are more prone to gastrointestinal conditions requiring acid suppression therapy, which positively influences demand for Lansoprazole.
Cost-Effective Treatment Preferences
With healthcare systems prioritizing budget-conscious choices, physicians opt for generics, fueling volume sales of GNP Lansoprazole DR.
Expanding Access in Emerging Markets
Improved healthcare infrastructure and increased awareness support broader distribution and consumption in regions like Asia, Latin America, and Africa.
Market Challenges
- Price Erosion: Entry of multiple generics leads to significant price drops.
- Prescribing Shifts: Increasing preference for other PPIs or H2 receptor antagonists in certain markets.
- Regulatory Scrutiny: Changes in labeling or manufacturing standards could impact supply and pricing.
- Patient Preferences: Preference for formulations with fewer dosing requirements or improved efficacy might influence market share.
Price Projection Analysis
Historical Pricing Trends
Historically, during patent exclusivity, brand-name Lansoprazole commanded premiums, but post-patent expiration, average prices for generics, including GNP Lansoprazole DR, declined sharply. In North America, the average retail price per pill for Lansoprazole 15 mg dropped by approximately 70-80% over five years following patent expiry (2).
Future Pricing Dynamics
The forecast indicates continued price erosion driven by intensified generic competition. However, several factors could stabilize or modestly increase prices:
- Market Consolidation: Larger pharmaceutical companies with increased manufacturing efficiencies may maintain relatively higher prices.
- Formulation Differentiation: Innovations in delayed-release mechanisms could command premium pricing.
- Supply Chain Constraints: Manufacturing challenges or raw material shortages may temporarily elevate prices.
- Regional Variations: Prices are expected to decline faster in mature markets like the US and Europe, while emerging markets may sustain higher prices due to lower generic penetration.
Projected Price Ranges (2023–2027)
| Year | Approximate Price per Unit (USD) | Key Drivers/Comments |
|---|---|---|
| 2023 | $0.10 – $0.20 | Post-patent expiration, high competition, low margins |
| 2024 | $0.09 – $0.18 | Continued price erosion, increased price competition |
| 2025 | $0.08 – $0.15 | Market saturation, possible small premium formulations |
| 2026 | $0.07 – $0.14 | Market consolidation, regional pricing disparities |
| 2027 | $0.07 – $0.12 | Stabilization in mature markets, growth in India/Asia |
Note: Prices are approximate and vary by region, formulation, and distribution channels.
Impact of Potential Regulatory and Market Factors
- Patent litigations or new formulations: Could temporarily stabilize or increase prices.
- Health policy changes: Favoring generics or biosimilar entries may accelerate decline.
- Market entry of alternative therapies: Could suppress demand and influence prices further.
Key Growth Opportunities
- Emerging Markets Expansion: Large populations and expanding healthcare access present growth opportunities.
- Formulation Innovation: Improved or combination formulations can command higher prices.
- Partnership and Alliances: Strategic collaborations can enhance distribution reach and market share.
Risks and Uncertainties
- Market Saturation: Excess supply could depress prices further.
- Pricing Regulations: Price controls in specific markets—particularly in Europe and Asia—may limit profitability.
- Generic Competition: Entry of new generic manufacturers or biosimilars could intensify price pressure.
- Efficacy and Safety Perception: Any negative perception could reduce demand.
Key Takeaways
- GNP Lansoprazole DR is positioned within a saturated but growing global market driven by the increasing prevalence of acid-related disorders.
- Price erosion is expected to persist, with prices decreasing by approximately 30-50% over the next five years in mature markets.
- The most notable growth opportunities lie in emerging markets and formulation innovations, potentially supporting higher price points.
- Strong competitive pressures necessitate strategic differentiation, possibly through formulation improvements or regional market focus.
- Market volatility examined through regulatory, economic, and healthcare policy factors should be closely monitored to refine strategic positioning.
FAQs
1. How does patent expiration influence GNP Lansoprazole DR pricing?
Patent expiration opens the market to generic competition, leading to significant price reductions; this trend has historically driven prices down by 70-80% within five years post-expiry, with further erosion expected as more Generics enter.
2. Which regions will sustain higher prices for Lansoprazole DR?
Emerging markets such as India, certain Southeast Asian countries, and Latin America may maintain higher prices due to lower generic penetration and limited regulatory price controls.
3. Can formulation innovations affect GNP Lansoprazole DR’s market viability?
Yes. Advanced delayed-release or combination formulations offering improved efficacy or convenience could command premium prices, offsetting some price erosion.
4. What are the main risks to future price stability?
Intense generic competition, regulatory price controls, and the emergence of alternative therapies threaten to further suppress prices.
5. How should companies strategize to enhance profitability?
Strategies include focusing on high-growth regions, investing in formulation improvements, forming strategic alliances, and navigating regulatory pathways effectively to differentiate products.
Sources
- MarketsandMarkets. Proton Pump Inhibitors Market by Product Type, Application, and Region – Global Forecast to 2026.
- IQVIA. Prescription trends for Lansoprazole post-patent expiry (various regional reports).
More… ↓
