Share This Page
Drug Price Trends for GLYBURIDE MICRO
✉ Email this page to a colleague
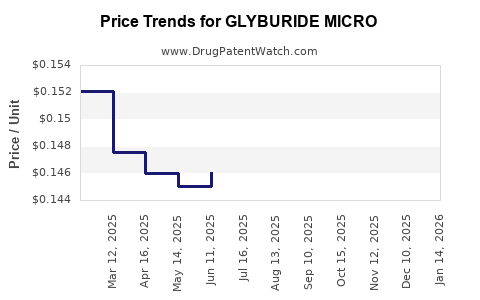
Average Pharmacy Cost for GLYBURIDE MICRO
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| GLYBURIDE MICRO 3 MG TABLET | 00093-8035-05 | 0.12504 | EACH | 2025-12-17 |
| GLYBURIDE MICRO 3 MG TABLET | 00093-8035-01 | 0.12504 | EACH | 2025-12-17 |
| GLYBURIDE MICRO 6 MG TABLET | 00093-8036-01 | 0.23059 | EACH | 2025-12-17 |
| GLYBURIDE MICRO 6 MG TABLET | 00093-8036-01 | 0.22538 | EACH | 2025-11-19 |
| GLYBURIDE MICRO 3 MG TABLET | 00093-8035-01 | 0.12695 | EACH | 2025-11-19 |
| GLYBURIDE MICRO 3 MG TABLET | 00093-8035-05 | 0.12695 | EACH | 2025-11-19 |
| GLYBURIDE MICRO 3 MG TABLET | 00093-8035-01 | 0.13029 | EACH | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Glyburide Micro
Introduction
Glyburide Micro, a generic formulation of the widely used oral antidiabetic medication, primarily targets type 2 diabetes management. It belongs to the sulfonylurea class, stimulating insulin secretion from pancreatic beta cells. As the global burden of diabetes rises—projected to affect 700 million individuals by 2045 [1]—the demand for affordable, effective oral hypoglycemics like Glyburide Micro continues to grow. This analysis explores market dynamics, competitive landscape, regulatory factors, and offers price projections to inform stakeholders ranging from manufacturers to investors.
Market Landscape
Global Diabetes Prevalence and Therapeutic Demand
The escalating prevalence of type 2 diabetes across developed and emerging markets sustains a significant demand for generics like Glyburide Micro. The International Diabetes Federation reports that the number of adults living with diabetes is growing annually, driven by lifestyle changes, urbanization, and aging populations [2]. Asia-Pacific nations, especially India and China, account for a sizeable share of this growth, creating fertile markets for affordable generics.
Current Market Adoption
Glyburide (also known as Glibenclamide in some regions) remains a cornerstone of therapy, especially in resource-limited settings where cost considerations predominate. The micro-dose formulation offers advantages in titration and minimizing hypoglycemia risk, thus appealing to both prescribers and patients. In highly regulated markets like the US, the drug operates within a robust generic landscape, constrained by patent expirations and increasing biosimilar competition.
Competitive Positioning
The primary competitors include other sulfonylureas (glipizide, glimepiride), DPP-4 inhibitors, and emerging novel agents. While newer classes boast favorable safety profiles, Glyburide Micro retains appeal due to its proven efficacy, low cost, and extensive clinical history.
Regulatory Environment
Regulatory approval for Glyburide Micro hinges on demonstrating bioequivalence to branded equivalents. As a generic, it faces fewer hurdles once approved, but quality standards must meet stringent pharmacopoeial guidelines. In jurisdictions where patent protections for innovator products have expired, market entry is facilitated.
Market Drivers and Constraints
Drivers
- Cost-effectiveness: Glyburide Micro's affordability makes it a major choice in low- and middle-income countries.
- Established efficacy: Decades of clinical data reinforce its therapeutic value.
- Growing diabetes epidemic: Continual rise in affected populations sustains demand.
Constraints
- Safety profile concerns: Risks of hypoglycemia and cardiovascular effects limit enthusiasm.
- Emergence of newer agents: SGLT2 inhibitors and GLP-1 receptor agonists challenge sulfonylureas’ positioning.
- Regulatory scrutiny: Quality assurance is imperative, especially for generics in regulatory tightening markets.
Pricing Dynamics
Current Pricing Landscape
In developed markets like the US, the average wholesale price (AWP) of generic Glyburide Micro ranges from $0.10 to $0.20 per tablet, reflecting competitive pressures and insurance negotiations [3]. In contrast, in developing nations, the retail price often hovers around $0.05 per tablet, driven by local manufacturing costs, subsidies, and patent expiry.
Factors Influencing Price Trends
- Generic market saturation: Increased competition drives downward prices.
- Manufacturing efficiencies: Large-scale production reduces unit costs.
- Regulatory and quality standards: Adherence to stringent standards can elevate costs but also assure quality perception.
- Supply chain dynamics: Import tariffs, distribution logistics, and geopolitical stability influence pricing.
Price Projections (2023-2030)
Based on current market trends, regulatory outlooks, and macroeconomic factors, the following projections are anticipated:
| Year | Expected Price Range per Tablet (USD) | Key Drivers/Notes |
|---|---|---|
| 2023 | $0.07 – $0.15 | Market saturation, intensified generic competition |
| 2024 | $0.07 – $0.14 | Further price erosion, increased manufacturing efficiencies |
| 2025 | $0.06 – $0.13 | Emerging markets leveraging local generics |
| 2026 | $0.06 – $0.12 | Price stabilization, regulatory pressures |
| 2027-2030 | $0.05 – $0.10 | Market consolidation, quality assurance costs, supply chain maturity |
These projections acknowledge continued commoditization, with prices trending downward, especially in high-volume markets, while maintaining margins through operational efficiencies.
Market Opportunities and Risks
Opportunities
- Expanding into Emerging Markets: Growing diabetes prevalence and limited access to high-cost therapies create expansion pathways.
- Formulation Innovations: Introducing extended-release or combination formulations can command premium pricing.
- Strategic Partnerships: Collaboration with local manufacturers enhances market penetration and reduces costs.
Risks
- Competitive Pricing Pressures: Entry of low-cost competitors can erode margins.
- Quality Concerns: Regulatory sanctions or recalls due to quality lapses threaten brand reputation.
- Regulatory Changes: Stringent approval standards or shifts in patent laws may delay market entry or extend time-to-market.
Conclusion
Glyburide Micro sustains a vital role in global diabetes management, especially in resource-limited settings where affordability is paramount. Market dynamics favor continued demand, with prices trending downward driven by generic proliferation and manufacturing efficiencies. Strategic positioning focusing on quality assurance, regional expansion, and formulation innovation offers pathways to sustain profitability.
Key Takeaways
- Market Demand: The global rise in type 2 diabetes continues to underpin sustained demand for affordable oral hypoglycemics like Glyburide Micro.
- Pricing Trends: Average prices are expected to decline between 2023 and 2030, with estimates ranging from $0.05 to $0.15 per tablet, influenced by competition and manufacturing factors.
- Competitive Landscape: Intensifying generic competition necessitates operational efficiencies and quality differentiation.
- Regulatory Impact: Strict adherence to quality standards is essential to maintain market access and avoid sanctions.
- Strategic Focus: Expansion into emerging markets, formulation innovations, and partnerships can optimize market share and margins.
FAQs
1. What factors influence the pricing of Glyburide Micro globally?
Pricing is primarily affected by manufacturing costs, competition among generics, regulatory standards, supply chain logistics, and regional economic conditions.
2. How does Glyburide Micro compare to newer antidiabetic agents in cost?
Glyburide Micro remains significantly more affordable than newer classes like SGLT2 inhibitors or GLP-1 receptor agonists, making it preferable in low-resource settings.
3. What challenges does Glyburide Micro face in maintaining market share?
Challenges include the emergence of newer therapeutics with better safety profiles, regulatory scrutiny over quality, and price competition from local generic manufacturers.
4. Are there opportunities for premium pricing with Glyburide Micro?
Yes. Innovations such as extended-release formulations, combination therapies, or improved delivery systems can command higher prices.
5. How will regulatory changes impact Glyburide Micro's market trajectory?
Enhanced quality standards may increase manufacturing costs but also bolster trust and market access. Conversely, stringent restrictions could delay approvals for new markets.
References
[1] International Diabetes Federation. IDF Diabetes Atlas, 10th Edition. 2021.
[2] International Diabetes Federation. "Diabetes Atlas 9th Edition," 2019.
[3] Medicare & Medicaid Services. ASP Drug Pricing Data, 2022.
More… ↓
