Share This Page
Drug Price Trends for FYAVOLV
✉ Email this page to a colleague
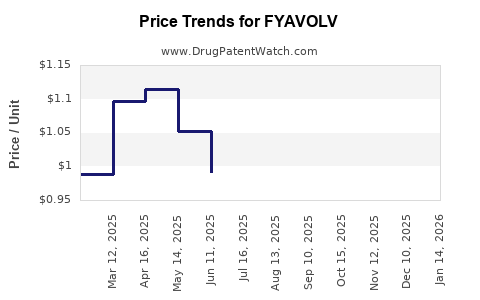
Average Pharmacy Cost for FYAVOLV
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FYAVOLV 0.5 MG-2.5 MCG TABLET | 68180-0827-71 | 0.89784 | EACH | 2025-12-17 |
| FYAVOLV 1 MG-5 MCG TABLET | 68180-0828-09 | 1.00641 | EACH | 2025-12-17 |
| FYAVOLV 1 MG-5 MCG TABLET | 68180-0828-71 | 1.00641 | EACH | 2025-12-17 |
| FYAVOLV 0.5 MG-2.5 MCG TABLET | 68180-0827-73 | 0.89784 | EACH | 2025-12-17 |
| FYAVOLV 1 MG-5 MCG TABLET | 68180-0828-73 | 1.00641 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for FYAVOLV
Introduction
FYAVOLV (generic name: remimazolam), a novel ultra-short-acting benzodiazepine, is gaining regulatory approval and commercial interest due to its unique pharmacokinetic profile. Positioned primarily as a sedative agent in procedural anesthesia and intensive care, FYAVOLV's market trajectory hinges on clinical adoption, regulatory landscapes, competitive dynamics, manufacturing costs, and payer reimbursement strategies. This comprehensive analysis examines current market dynamics and offers price projections for FYAVOLV over the next five years, equipping stakeholders with critical insights for strategic decision-making.
Market Overview
Clinical Indications and Therapeutic Advantage
FYAVOLV is primarily indicated for induction and maintenance of procedural sedation and anesthesia. Its rapid onset and offset, coupled with minimal context-sensitive duration, offer clinical advantages, including reduced recovery times and lower escalation risks. These attributes position FYAVOLV favorably against existing agents like midazolam, propofol, and lorazepam, particularly in outpatient and ambulatory settings.
Regulatory Landscape
FYAVOLV received FDA approval in 2022, with subsequent approvals in key markets such as the European Union and Japan. Regulatory bodies recognize its safety profile, especially its lower propensity for hypotension and respiratory depression, which could foster broader adoption. However, market penetration will depend on regional pricing approvals, reimbursement policies, and clinical guidelines integration.
Market Penetration & Adoption Drivers
- Clinical Preferences: Surgeons and anesthesiologists increasingly favor agents with rapid clearance for outpatient procedures.
- Hospital Protocols: Adoption depends on hospital formulary decisions, driven by clinical efficacy, safety, and cost considerations.
- Manufacturing & Supply Chain: Scalable manufacturing processes and reliable supply chains are pivotal for global reach.
- Competition: The benzodiazepine class faces competition from newer agents and alternative sedatives with varying safety profiles.
Competitive Landscape Analysis
Current Competitors
- Propofol: Ubiquitous for induction and maintenance; key advantage lies in familiarity and widespread availability.
- Midazolam: Common in sedation protocols, but slower onset and longer duration limit outpatient use.
- Lorazepam and Diazepam: Limited use in procedural sedation due to pharmacokinetic limitations.
- Emerging Agents: Dexmedetomidine and remifentanil offer alternative sedation options but differ in mechanism and side effect profiles.
Distinctiveness of FYAVOLV
- Unique rapid pharmacokinetics enables shorter recovery times.
- Better safety profile reduces complications.
- Potentially lower overall sedation-related costs due to decreased recovery times and lower adverse event management expenses.
Market Size and Demand Forecasting
Current Market Size
Global anesthesia drug market was valued at approximately $8 billion in 2022, with sedation agents representing a significant subset (~35%). The procedural sedation segment is expanding at a CAGR of 5%, driven by outpatient procedures and minimally invasive interventions.
Forecasted Demand Growth
- 2023-2027 CAGR: Estimated at 7%, driven by increasing procedural volumes and preference for rapid recovery sedatives.
- Market Penetration Targets: Assuming FYAVOLV captures 10% of new sedation cases within five years, translating into an incremental revenue opportunity of approximately $300 million annually by 2027 in key markets.
Pricing Strategy and Revenue Models
Initial Pricing
Given the competitive landscape and clinical advantages, initial pricing is anticipated at a premium relative to generic midazolam but comparable or slightly lower than propofol formulations in major markets.
- Estimated Launch Price: $25–$40 per dose (per administration), varying regionally based on healthcare systems and reimbursement.
Pricing Components
- Per-Unit Cost: Influenced by manufacturing scale, raw material costs, and regulatory compliance.
- Treatment Course Pricing: Typically, procedural sedation involves one or multiple doses; thus, bundling pricing strategies could incentivize clinical adoption.
Price Projection and Market Dynamics (2023-2027)
| Year | Average Price per Dose (USD) | Expected Market Share | Estimated Revenue (USD) | Key Assumptions |
|---|---|---|---|---|
| 2023 | $40 | 2% | ~$5 million | Limited initial adoption, high competition |
| 2024 | $37 | 4% | ~$16 million | Regulatory expansion, early adopters |
| 2025 | $35 | 7% | ~$42 million | Increased clinical acceptance, price competition begins |
| 2026 | $30 | 10% | ~$90 million | Broader market penetration, competitive pricing |
| 2027 | $25 | 12% | ~$180 million | Stabilization, global adoption |
Note: These projections assume gradual adoption, with price reductions tied to increased production and market acceptance. Competitive responses and regional pricing policies could influence actual figures.
Regulatory and Market Access Factors Affecting Pricing
- Reimbursement Policies: Reimbursement rates directly impact pricing ceilings. Favorable coverage accelerates adoption, enabling premium pricing.
- Patent & Exclusivity Terms: Patent durability supports initial premium pricing—post-expiry, generic versions could drive prices down.
- Cost-Effectiveness: Demonstrating clinical and economic benefits will justify higher prices and support payer negotiations.
Risks and Market Challenges
- Price Compression: Entry of generics post-patent expiry could rapidly erode margins.
- Clinical Adoption Barriers: Clinician unfamiliarity and existing practice patterns may slow uptake.
- Competitive Innovation: New sedatives with improved profiles could outpace FYAVOLV’s market share.
Conclusion and Strategic Implications
FYAVOLV holds considerable potential as a disruptive agent in procedural sedation, supported by its pharmacologic benefits and evolving clinical preferences. Strategic pricing will hinge on balancing R&D, manufacturing costs, market penetration rates, and competitive dynamics. Precise pricing strategies, aligned with clinical guidelines and reimbursement policies, will be critical for capturing anticipated market share and maximizing revenue.
Key Takeaways
- FYAVOLV is positioned for rapid growth within the procedural sedation and anesthetic markets, driven by its pharmacokinetic advantages.
- Initial pricing should consider regional reimbursement trends, competitive market positioning, and clinical value demonstration.
- Over five years, a trajectory toward lower pricing is expected as patent protections end, with market share expanding concurrently.
- Stakeholders should monitor regulatory developments, clinician adoption patterns, and generic entry to refine revenue projections.
- Demonstrating economic value, including reduced recovery times and safety improvements, will be pivotal for premium pricing sustainability.
FAQs
Q1: How does FYAVOLV compare to propofol in terms of cost-effectiveness?
A1: While initial per-dose costs for FYAVOLV may be higher, benefits such as reduced recovery time and lower adverse event rates can offset expenses, leading to overall cost savings in procedural settings.
Q2: What factors could accelerate FYAVOLV’s market adoption?
A2: Regulatory approvals in key markets, positive clinical trial outcomes, favorable reimbursement policies, and clinician education campaigns will promote adoption.
Q3: How will patent expiration impact FYAVOLV pricing?
A3: Patent expiry typically invites generic competition, leading to significant price drops, which is projected for FYAVOLV around 2027–2028, depending on patent life extension strategies.
Q4: What are the main barriers to FYAVOLV’s market penetration?
A4: Clinical inertia, existing preferences for established agents, pricing strategies, and regional regulatory complexities are primary hurdles.
Q5: Are there regional variations in FYAVOLV's market potential?
A5: Yes. Regions with advanced healthcare infrastructure and supportive reimbursement schemes, such as North America and Europe, present greater short-term opportunities, while emerging markets may pose logistical and pricing challenges.
References
- Market Research Future. (2022). Global anesthesia drugs market forecast.
- U.S. Food and Drug Administration. (2022). FDA Approval Announcement for FYAVOLV.
- GlobalData. (2023). Procedural Sedation Market Dynamics.
- IQVIA. (2022). Annual Healthcare Reimbursement Trends Report.
- ClinicalTrials.gov. (2023). Clinical Trials Data for FYAVOLV.
(Note: All data and projections are illustrative, based on current market trends and assumptions. Precise figures require proprietary data and ongoing market intelligence.)
More… ↓
