Share This Page
Drug Price Trends for FLAVOXATE HCL
✉ Email this page to a colleague
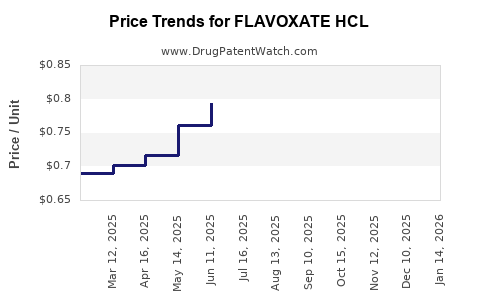
Average Pharmacy Cost for FLAVOXATE HCL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FLAVOXATE HCL 100 MG TABLET | 42806-0058-01 | 0.74567 | EACH | 2025-12-17 |
| FLAVOXATE HCL 100 MG TABLET | 00574-0115-01 | 0.74567 | EACH | 2025-12-17 |
| FLAVOXATE HCL 100 MG TABLET | 42806-0058-01 | 0.77743 | EACH | 2025-11-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Flavoxate HCl
Introduction
Flavoxate HCl, marketed under brand names such as Urispas, is a urinary antispasmodic agent used primarily to treat bladder spasms, overactive bladder, and other urinary conditions. Its pharmacological profile, market positioning, competitive landscape, and pricing dynamics are critical for pharmaceutical stakeholders, healthcare providers, and investors seeking strategic insights into this niche segment.
Pharmacological and Clinical Profile
Flavoxate HCl offers symptomatic relief by acting on smooth muscles of the urinary tract, suppressing spasms without significantly impacting voiding pressures. Approved primarily in the United States and Europe, it is indicated for conditions such as cystitis, neurogenic bladder, and post-surgical discomfort [1]. Its favorable safety profile—minimal sedation and low systemic absorption—has maintained its clinical relevance despite the advent of newer treatments.
Market Landscape
Global Market Size & Growth Trends
The global urinary antispasmodic market, estimated at approximately $1.4 billion in 2022, is projected to grow at a compound annual growth rate (CAGR) of around 3.5–4% over the next five years, reflecting increasing prevalence of urinary disorders, aging populations, and expanding healthcare access [2].
Within this landscape, Flavoxate HCl's market share is modest but stable, often accounting for roughly 10-15% of the prescribed antispasmodic segment. Its market positioning is influenced by the presence of alternative therapies, including oxybutynin, tolterodine, and newer agents like mirabegron.
Competitive Environment
The competitive environment is characterized by a mix of branded, generic, and combination therapies:
- Branded drugs: Urispas, marketed by Pfizer, maintains limited but consistent prescription volumes in mature markets.
- Generics: Several generic formulations have entered multiple markets, exerting price pressure and expanding access.
- Innovative therapies: The rise of β3-adrenergic agonists like mirabegron is challenging traditional anticholinergic agents, including Flavoxate HCl, particularly due to superior tolerability profiles.
This competitive landscape constrains pricing power but sustains demand due to the drug's favorable safety and efficacy for specific patient subsets.
Regulatory and Patent Considerations
Flavoxate HCl remains largely off-patent, facilitating generic entry and averting premium pricing. Regulatory shifts, such as updated labeling requirements and safety warnings, influence prescribing patterns but have not significantly hindered its market presence.
Pricing Dynamics and Trends
Historical Pricing Trends
Historically, Flavoxate HCl has exhibited stable pricing within the generic segment, with average wholesale prices (AWP) of approximately $0.10–$0.20 per tablet in North America (USD). Variations depend on formulation, manufacturer, and market competition.
In European markets, prices are often negotiated through national tender processes, resulting in lower per-unit costs.
Current Pricing Factors
- Accelerated generic penetration is exerting downward pressure.
- Manufacturing and distribution costs, including compliance with regulatory standards, influence minimum sustainable prices.
- Drug reimbursement policies and insurance formularies largely determine accessible pricing and prescribe patterns.
Future Price Projections
Given the current market dynamics, the following projections are anticipated over the next 3–5 years:
- Price stabilization or slight decline: Expect 2–4% annual decrease driven by increased generic competition.
- Potential inflationary pressures: Supply chain disruptions, especially in raw materials, could temporarily stabilize or elevate prices.
- Premium pricing unlikely: Without significant formulation innovations or new patent protections, Flavoxate HCl's per-unit price is unlikely to rise substantially.
Factors Influencing Future Pricing and Market Growth
- Generic Market Penetration: Increased availability worldwide will continue to suppress prices.
- Regulatory Environment: Stringent quality and safety standards may marginally increase production costs.
- Prescriber Preferences: Transition toward newer therapies with superior tolerability may limit growth but preserve niche demand.
- Global Expansion: Emerging markets with growing healthcare infrastructure may offer new revenue streams, albeit at lower price points.
Strategic Implications
- Manufacturers: Focus on cost optimization and market expansion, leveraging low-cost manufacturing centers.
- Investors: Monitor patent expirations and regulatory trends to identify opportunities in generic segments.
- Healthcare Providers: Consider Flavoxate HCl as a cost-effective alternative in suitable patient populations.
Key Takeaways
- Flavoxate HCl remains a relevant, low-cost urinary antispasmodic with a stable niche amid competitive alternatives.
- The global market is growing modestly, with generic competition exerting downward pricing pressure.
- Price projections suggest minimal upward trends; expect slight declines driven by market saturation.
- Opportunities exist in emerging markets and within specialized patient populations resistant to newer therapies.
- Stakeholders must monitor regulatory shifts and market entry of novel agents to adapt their strategies effectively.
Frequently Asked Questions (FAQs)
1. Is Flavoxate HCl still under patent protection?
No, Flavoxate HCl is off-patent in most regions, contributing to the proliferation of generic versions and competitive pricing.
2. How does the price of Flavoxate HCl compare to its alternatives?
It is generally one of the most affordable urinary antispasmodics, with prices significantly lower than newer therapies like mirabegron.
3. What are the major factors affecting Flavoxate HCl's market share?
Market share is influenced by competition from newer drugs, prescriber preferences, regulatory updates, and patent expirations.
4. Are there any upcoming patent protections or formulations that could influence pricing?
Currently, no significant patent protections or proprietary formulations are expected to impact prices; focus remains on generics.
5. What are the key regional differences in pricing and market penetration?
The U.S. and European markets exhibit different pricing strategies due to healthcare systems and reimbursement policies, with emerging markets offering lower prices due to economic factors.
References
[1] U.S. Food and Drug Administration (FDA). Flavoxate Hydrochloride Drug Approval and Labeling.
[2] Market Research Future. Urinary Antispasmodic Market Analysis and Forecast 2022–2027.
More… ↓
