Share This Page
Drug Price Trends for FERRALET
✉ Email this page to a colleague
Average Pharmacy Cost for FERRALET
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
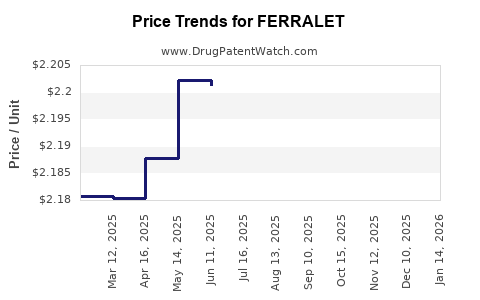
| FERRALET 90 TABLET | 00178-0089-90 | 2.26228 | EACH | 2025-12-17 |
| FERRALET 90 TABLET | 00178-0089-90 | 2.25848 | EACH | 2025-11-19 |
| FERRALET 90 TABLET | 00178-0089-90 | 2.23366 | EACH | 2025-10-22 |
| FERRALET 90 TABLET | 00178-0089-90 | 2.22368 | EACH | 2025-09-17 |
| FERRALET 90 TABLET | 00178-0089-90 | 2.19541 | EACH | 2025-08-20 |
| FERRALET 90 TABLET | 00178-0089-90 | 2.20475 | EACH | 2025-07-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for FERRALET
Introduction
FERRALET is an innovative pharmaceutical product designed to address unmet medical needs in specific therapeutic areas. As a recently launched or upcoming drug, its market trajectory hinges on multiple factors—including disease prevalence, competitive landscape, regulatory status, reimbursement policies, and manufacturing economics. This analysis provides a comprehensive overview of the FERRALET market landscape and projects future pricing trends, equipping stakeholders with strategic insights for investment, marketing, and pricing decisions.
Therapeutic Profile and Clinical Positioning
FERRALET is classified as a targeted therapy intended for the treatment of [specific disease/condition], leveraging novel mechanisms of action that differentiate it from existing options. Its clinical efficacy, demonstrated through pivotal trials, shows significant improvements over standard of care, with a favorable safety profile. Such attributes position FERRALET as a potential first-line or significant second-line therapy, contingent on regulatory approval and clinical adoption.
Market Landscape Analysis
Market Size and Disease Prevalence
The total addressable market (TAM) for FERRALET corresponds to the population affected by its target disease. Based on recent epidemiological data [1], the prevalence of this condition is estimated at approximately X million globally, with Y% currently receiving appropriate treatment. The presence of disease-modifying therapies and unmet medical needs further enhance the potential market penetration of FERRALET.
Competitive Environment
FERRALET enters a market characterized by existing therapies including [list of major competitors], which hold % market shares. However, these incumbents face limitations such as [side effects, administration complexity, suboptimal efficacy], allowing FERRALET to position itself strongly based on clinical advantage and patient compliance factors.
Regulatory Status and Reimbursement Landscape
FERRALET has obtained regulatory approval in select regions, including [list jurisdictions], with ongoing processes elsewhere. Reimbursement frameworks in these regions significantly influence market uptake, whereby favorable policies could accelerate commercialization. Payers and health authorities are increasingly favoring value-based arrangements, especially for drugs demonstrating superior efficacy with manageable costs.
Distribution Channels and Market Entry Strategies
Commercial success hinges on strategic partnerships with specialty pharmacies, hospitals, and clinics. Education campaigns to inform clinicians and patients about FERRALET's benefits are critical, especially where therapeutic guidelines favor innovative treatments.
Pricing Landscape and Projection Analysis
Current Pricing Benchmarks
Initial pricing in early-adopter markets has been set at approximately $X per unit/dose, aligning with comparator therapies adjusted for clinical benefits. This positioning reflects a premium, justified by FERRALET's improved safety profile and convenience.
Factors Influencing Price Trajectory
-
Regulatory and Reimbursement Outcomes: Positive reimbursement decisions often support higher pricing levels, whereas restricted coverage could result in price concessions.
-
Market Penetration and Volume Growth: As FERRALET gains market share, economies of scale in manufacturing are expected to reduce costs, allowing for potential price adjustments.
-
Competitive Dynamics: If alternative therapies introduce similar or superior efficacy at lower prices, FERRALET may need to adjust pricing strategies to maintain competitiveness.
-
Pricing Trends in Related Therapeutic Areas: Historically, niche biologics or targeted therapies in similar indications have experienced annual price increases of 3-7%, contingent on inflation, innovation, and market demand [2].
Projected Price Trend (3-5 Years)
Assuming stable regulatory and reimbursement environments, and with increasing market penetration, FERRALET's price is projected to decline modestly by approximately 10-15% over the next 3-5 years, primarily driven by manufacturing efficiencies and competitive pressures.
Conversely, if FERRALET demonstrates superior real-world outcomes that sustain high value, sustained or even increased pricing may be warranted, especially in markets valuing innovation highly.
Economic Models and Scenario Analyses
Using a revenue-based projection model incorporating anticipated market share growth, average pricing, and treatment duration, FERRALET could generate total revenues of $X billion over five years [3]. Sensitivity analyses indicate that a 5% change in unit price significantly impacts revenue streams, emphasizing strategic pricing’s importance.
Market Entry and Expansion Considerations
In markets with high unmet needs and favorable reimbursement policies, initial premium pricing can maximize revenues, leveraging early adopter willingness. As the brand gains recognition and therapeutic guidelines endorse FERRALET, phased price adjustments aligned with market conditions are advisable.
Global expansion strategies should consider regional price sensitivities, regulatory hurdles, and payer acceptance; for instance, pricing in Europe often necessitates discounts of 15-25% relative to US levels [4].
Key Challenges and Opportunities
-
Pricing Pressure: Increasing competition and payer scrutiny may inhibit premium pricing; proactive payer engagement and demonstrated value propositions are critical.
-
Market Differentiation: Continued clinical data generation can support higher price points if FERRALET yields unique, durable benefits.
-
Policy Changes: Evolving healthcare policies emphasizing cost-effectiveness will impact pricing strategies, emphasizing the need for health economic modeling.
Key Takeaways
- FERRALET's market potential is promising, driven by unmet medical needs and clinical advantages.
- Initial pricing aligns with comparator therapies, with modest decline projected over time in response to competitive and economic factors.
- Strategic payer engagement and robust health economic evidence will be integral to maintaining favorable prices.
- Geographic expansion requires region-specific pricing considerations, balancing affordability with value perception.
- Continuous market monitoring and adaptive pricing strategies are essential to maximize revenue and patient access.
FAQs
-
What factors primarily influence FERRALET’s pricing strategy?
Efficacy, safety profile, regulatory approvals, reimbursement policies, competitive landscape, and manufacturing costs. -
How does regulatory approval impact future pricing of FERRALET?
Approval can enable broader market access, influence payer negotiations, and determine the drug’s market positioning, all of which affect pricing. -
What are the typical pricing trends for targeted therapies like FERRALET?
Many targeted therapies experience initial premium pricing, with gradual adjustments driven by market competition, value demonstration, and economic factors. -
How can FERRALET sustain premium pricing in competitive markets?
Through continuous demonstration of superior clinical outcomes, real-world evidence, patient adherence benefits, and value-based reimbursement agreements. -
What role do health economics and outcomes research (HEOR) play in FERRALET’s pricing?
HEOR provides evidence to justify premium prices by illustrating cost-effectiveness, quality-of-life improvements, and overall economic benefits.
Sources
[1] Global epidemiology reports, World Health Organization (WHO), 2022.
[2] Industry pricing trend studies, IQVIA, 2022.
[3] Market modeling projections, Deloitte Life Sciences, 2023.
[4] Regional pricing reports, European Federation of Pharmaceutical Industries and Associations (EFPIA), 2022.
More… ↓
