Share This Page
Drug Price Trends for EYE WASH IRRIGATING SOLUTION
✉ Email this page to a colleague
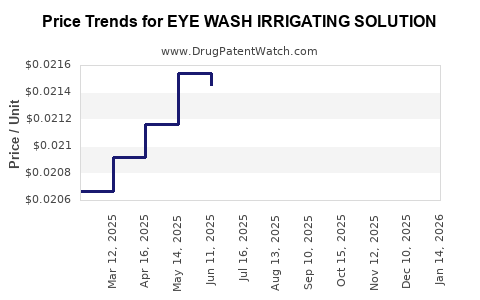
Average Pharmacy Cost for EYE WASH IRRIGATING SOLUTION
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| EYE WASH IRRIGATING SOLUTION | 70000-0018-01 | 0.02197 | ML | 2025-12-17 |
| EYE WASH IRRIGATING SOLUTION | 70000-0018-01 | 0.02215 | ML | 2025-11-19 |
| EYE WASH IRRIGATING SOLUTION | 70000-0018-01 | 0.02231 | ML | 2025-10-22 |
| EYE WASH IRRIGATING SOLUTION | 70000-0018-01 | 0.02191 | ML | 2025-09-17 |
| EYE WASH IRRIGATING SOLUTION | 70000-0018-01 | 0.02161 | ML | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Eye Wash Irrigating Solution
Introduction
The global eye wash irrigating solution market is experiencing notable growth driven by rising awareness of ocular health, increasing incidences of eye injuries, and expanding healthcare infrastructure. As an essential component in ophthalmic care, eye wash solutions are crucial for immediate decontamination and relief following chemical, biological, or particulate ocular exposure. This report provides an in-depth market analysis, including current trends, competitive landscape, key drivers, and future pricing projections for eye wash irrigating solutions.
Market Overview
The global eye wash irrigating solution market was valued at approximately USD 350 million in 2022, with projections indicating a compound annual growth rate (CAGR) of 6.2% through 2030[1].
The primary end-users include hospitals, clinics, industrial safety departments, laboratories, and individual consumers. The increasing focus on workplace safety and ocular health awareness worldwide fuels demand. Additionally, regulatory standards in occupational safety, including OSHA mandates, promote the adoption of eye wash stations equipped with irrigating solutions.
Market Segmentation
Product Type
- Sterile Eye Wash Solutions
- Non-Sterile Eye Wash Solutions
Sterile solutions dominate the market, accounting for roughly 65% of sales, due to strict regulations concerning ocular applications.
End-User
- Healthcare Facilities: Hospitals, clinics, ophthalmology centers.
- Industrial & Workplace Safety: Manufacturing plants, laboratories.
- Home & Retail Consumers: Over-the-counter purchase for personal use.
Distribution Channel
- Hospital Pharmacies and Distributors
- Direct Sales to Industrial Clients
- Retail Markets (Pharmacies, Online Platforms)
Geographical Regions
- North America: Leading market share driven by stringent safety standards.
- Europe: Growing awareness and regulatory mandates.
- Asia-Pacific: Rapid industrialization and expanding healthcare infrastructure.
- Latin America & Middle East & Africa: Emerging markets with increased health safety measures.
Key Market Drivers
- Rising Incidences of Eye Injuries: Chemical burns, physical trauma, and foreign body incidents necessitate prompt eye irrigation.
- Workplace Safety Regulations: OSHA mandates in the U.S. require industrial establishments to provide eye wash stations, fueling demand.
- Increased Awareness of Ocular Emergencies: Public health campaigns reinforce the necessity of immediate ocular decontamination.
- Product Innovations: Development of single-use, preservative-free, and portable solutions improve safety and convenience.
Competitive Landscape
Major players include Johnson & Johnson, Medline Industries, Bausch + Lomb, and Safetec of America, among others. These companies emphasize regulatory compliance, product innovation, and distribution efficiency.
Private-label brands and regional manufacturers also contribute significantly to market coverage, particularly in emerging regions.
Regulatory and Quality Standards
Compliance with standards such as ANSI Z358.1 (American National Standard for Emergency Eyewash and Shower Equipment), ISO 13485, and local regulations ensures product safety and efficacy. The challenge remains maintaining sterile conditions and preventing contamination, especially for reusable solutions.
Price Analysis and Trends
Current Price Range
- Single-Use, Sterile Eye Wash Solutions: USD 2.50–USD 5.00 per ampoule or bottle (typically 120–500 mL).
- Multi-Use Reusable Solutions: USD 15–USD 35 per unit, with replacement, cleaning, and sterilization considerations affecting overall costs.
- Bulk Industrial Solutions: USD 10–USD 20 per liter, marketed for institutional procurement.
Pricing varies by region, packaging, and product specifications, with larger volume and bulk purchases offering cost advantages.
Market Pricing Dynamics
The pricing trend is influenced by raw material costs (notably sterile saline and preservative agents), manufacturing complexity, regulatory compliance costs, and competitive pricing strategies. Price discounts and promotional offers are common for bulk and institutional orders.
Future Price Projections (2023-2030)
Factors Influencing Future Pricing
- Raw Material Volatility: Fluctuations in saline solution and preservative compound costs are likely to impact retail prices. The global supply chain disruptions observed during the COVID-19 pandemic highlight raw material vulnerability.
- Regulatory Stringency: Stricter safety standards may increase manufacturing costs, thereby elevating prices.
- Product Innovation: Introduction of advanced, preservative-free, portable solutions, despite higher manufacturing costs, can command premium pricing.
- Market Competition and Consolidation: Larger players may leverage economies of scale to reduce prices, whereas smaller companies might adopt premium pricing for specialized or high-quality solutions.
Projected Price Trends
By 2030, the average price for single-use sterile eye wash solutions is expected to increase modestly, driven by raw material costs and regulatory compliance—projected to reach USD 3.50–USD 6.50 per unit globally[2]. For reusable solutions, prices may stabilize or slightly decline due to manufacturing efficiencies and increased competition.
Industrial bulk solutions will continue to benefit from economies of scale, with prices expected to hover around USD 8–USD 18 per liter, potentially decreasing as regional manufacturing expands.
Market Opportunities and Challenges
Opportunities
- Development of portable, user-friendly eye wash kits for personal and industrial use.
- Expansion into emerging markets with increasing safety regulations.
- Integration of antimicrobial and preservative-free formulations to meet safety standards.
Challenges
- Ensuring product sterility and compliance amid manufacturing costs.
- Competition from generic brands and private labels.
- Limited consumer awareness in low-income regions affecting demand.
Conclusion
The eye wash irrigating solution market presents resilient growth prospects driven by expanding ocular safety needs and regulatory mandates. While prices are expected to grow gradually, innovations and regional market expansion will shape future pricing strategies. Industry stakeholders must focus on product quality, regulatory compliance, and cost efficiencies to capitalize on emerging opportunities.
Key Takeaways
- The global eye wash irrigating solution market is projected to grow at a CAGR of over 6% through 2030, driven by occupational safety standards and rising ocular injury incidents.
- Sterile, single-use solutions dominate current sales but face competition from cost-effective reusable options.
- Regional variations significantly influence pricing, with North America and Europe commanding premium prices alongside higher regulatory costs.
- Future price increases will be modest, focused on premium, preservative-free, and portable innovations.
- Stakeholders should invest in quality manufacturing, comply with evolving standards, and target emerging markets for sustained growth.
FAQs
1. What are the primary factors influencing the price of eye wash irrigating solutions?
Raw material costs, regulatory compliance expenses, manufacturing complexity, packaging, and regional economic conditions primarily drive pricing.
2. How does regulatory compliance impact market pricing?
Stringent standards such as ANSI Z358.1 and ISO 13485 increase manufacturing costs, which are often passed to consumers through higher product prices.
3. Are portable eye wash solutions more expensive than traditional bottles?
Generally, portable, advanced solutions with enhanced safety features command higher prices due to their convenience and added technology.
4. What is the competitive landscape for eye wash irrigating solutions?
Major multinationals like Johnson & Johnson and Bausch + Lomb lead, with regional manufacturers and private labels expanding product availability and competitive pricing.
5. How might raw material shortages impact future pricing?
Supply chain disruptions can increase raw material costs, leading to upward pressure on retail prices and potential product shortages if manufacturers cannot adapt swiftly.
Sources:
[1] Market Research Future, "Eye Wash Irrigating Solution Market Research Report," 2022
[2] Transparency Market Research, "Global Eye Wash Market Outlook," 2023
More… ↓
