Drug Price Trends for EPLERENONE
✉ Email this page to a colleague
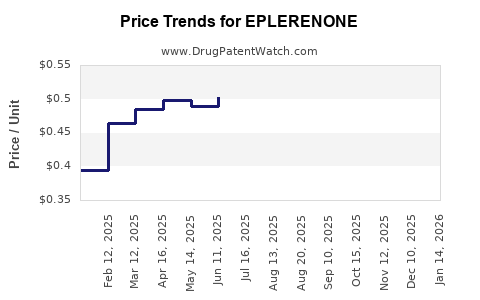
Average Pharmacy Cost for EPLERENONE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| EPLERENONE 25 MG TABLET | 16571-0173-09 | 0.36124 | EACH | 2025-12-17 |
| EPLERENONE 25 MG TABLET | 16729-0293-10 | 0.36124 | EACH | 2025-12-17 |
| EPLERENONE 25 MG TABLET | 31722-0049-90 | 0.36124 | EACH | 2025-12-17 |
| EPLERENONE 25 MG TABLET | 16571-0173-03 | 0.36124 | EACH | 2025-12-17 |
| EPLERENONE 25 MG TABLET | 16729-0293-15 | 0.36124 | EACH | 2025-12-17 |
| EPLERENONE 50 MG TABLET | 69367-0308-30 | 0.49674 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Best Wholesale Price for EPLERENONE
| Drug Name | Vendor | NDC | Count | Price ($) | Price/Unit ($) | Unit | Dates | Price Type |
|---|---|---|---|---|---|---|---|---|
| EPLERENONE 25MG TAB | AvKare, LLC | 69367-0307-09 | 90 | 43.04 | 0.47822 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| EPLERENONE 25MG TAB | AvKare, LLC | 69367-0307-30 | 30 | 10.24 | 0.34133 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| EPLERENONE 25MG TAB | AvKare, LLC | 42291-0454-30 | 30 | 9.73 | 0.32433 | EACH | 2024-01-12 - 2028-06-14 | FSS |
| EPLERENONE 50MG TAB | AvKare, LLC | 69367-0308-09 | 90 | 69.56 | 0.77289 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| EPLERENONE 25MG TAB | AvKare, LLC | 42291-0454-90 | 90 | 30.32 | 0.33689 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| EPLERENONE 50MG TAB | AvKare, LLC | 69367-0308-30 | 30 | 14.34 | 0.47800 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| >Drug Name | >Vendor | >NDC | >Count | >Price ($) | >Price/Unit ($) | >Unit | >Dates | >Price Type |
Market Analysis and Price Projections for Eplerenone
More… ↓
