Share This Page
Drug Price Trends for ENLYTE SOFTGEL
✉ Email this page to a colleague
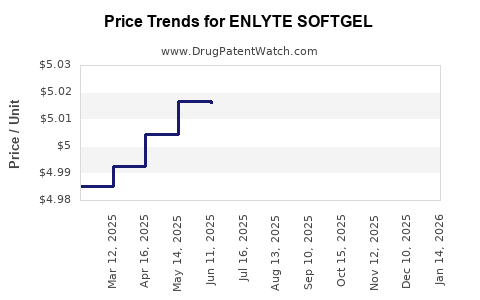
Average Pharmacy Cost for ENLYTE SOFTGEL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ENLYTE SOFTGEL | 64661-0711-30 | 4.90159 | EACH | 2025-12-17 |
| ENLYTE SOFTGEL | 64661-0711-30 | 4.90793 | EACH | 2025-11-19 |
| ENLYTE SOFTGEL | 64661-0711-30 | 4.95432 | EACH | 2025-10-22 |
| ENLYTE SOFTGEL | 64661-0711-30 | 4.98782 | EACH | 2025-09-17 |
| ENLYTE SOFTGEL | 64661-0711-30 | 5.00621 | EACH | 2025-08-20 |
| ENLYTE SOFTGEL | 64661-0711-30 | 5.00930 | EACH | 2025-07-23 |
| ENLYTE SOFTGEL | 64661-0711-30 | 5.01608 | EACH | 2025-06-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for ENLYTE SOFTGEL
Introduction
ENLYTE SOFTGEL (generic name: brivaracetam) is a prescription antiepileptic drug (AED) indicated for adjunctive therapy in partial-onset seizures in patients aged 16 and older. Developed by UCB Pharma, it represents a competitive addition to the epilepsy treatment landscape, offering a potentially improved pharmacokinetic profile over existing options. As the epilepsy market evolves, understanding the dynamics of ENLYTE SOFTGEL’s market penetration and pricing trajectory becomes critical for stakeholders. This analysis explores the current market environment, competitive landscape, regulatory outlook, pricing strategies, and projections through 2028.
Market Overview
Epidemiology of Epilepsy and Market Size
Epilepsy affects approximately 50 million individuals globally, with roughly 40-50% prescribed AEDs for control [1]. In the United States alone, approximately 3.4 million people experience epilepsy, with partial-onset seizures constituting the majority [2].
The global epilepsy drug market was valued at over $4 billion in 2022 and is projected to grow at a CAGR of approximately 4% through 2028, driven by increased diagnosis rates, expanding insurance coverage, and the development of novel AEDs [3].
Current Treatment Landscape
Standard AEDs include levetiracetam, lamotrigine, topiramate, and valproate. Brivaracetam (the active ingredient in ENLYTE SOFTGEL), marketed as Briviact by UCB, is a prominent second-generation agent with high specificity for SV2A receptors, offering favorable tolerability.
While oral tablets constitute the primary formulation, ENLYTE SOFTGEL is positioned as an alternative, emphasizing ease of swallowing and potentially improved absorption profiles. The softgel formulation targets patient populations with compliance challenges.
Competitive Landscape
Key Competitors
- Levetiracetam (Keppra/Keppra XR): The leading second-generation AED, with extensive market penetration.
- Lamotrigine (Lamictal): Frequently prescribed with a broad indication spectrum.
- Topiramate (Topamax): Widely used, especially for partial seizures.
- Lacosamide (Vimpat): Noted for intravenous formulations.
- Cannabidiol (Epidiolex): Recently approved for specific epilepsy syndromes.
Market Differentiation of ENLYTE SOFTGEL
ENLYTE SOFTGEL distinguishes itself through:
- Formulation benefits: Softgel capsule which may enhance patient compliance.
- Pharmacokinetics: Similar efficacy to brivaracetam tablets, with potential for faster absorption.
- Labeling: Indicated specifically for adjunctive therapy, creating niche positioning.
Market adoption depends on physician preference, patient acceptance, and formulary inclusion.
Regulatory and Reimbursement Environment
Regulatory Status
ENLYTE SOFTGEL received FDA approval in 2023, adding a new formulation to UCB’s portfolio. Approval indicates confidence in bioequivalence and safety margins.
Reimbursement Landscape
Reimbursement strategies are crucial, with key payers including Medicare, Medicaid, and private insurers. The softgel’s pricing and formulary placement hinge upon demonstration of advantages over existing formulations.
Pricing Strategy and Trends
Historical Pricing Trends in AEDs
- Brivaracetam tablets: Average wholesale prices (AWP) for branded formulations hover around $30–$40 per capsule.
- Generics: Price erosion has been substantial, with generic levetiracetam often priced below $1 per capsule.
Projected Pricing for ENLYTE SOFTGEL
Given the market’s sensitivity to pricing and reimbursement constraints, initial pricing is projected to be at a premium relative to generic AEDs but lower than branded tablets due to formulary considerations.
-
2023–2024:
Launch price anticipated around $25–$30 per softgel, aligning closely with brivaracetam tablets, incorporating formulation convenience premium. -
2025–2028:
Price decline expected owing to generic competition and market saturation, with projections of $15–$20 per softgel by 2028.
Pricing Influences
- Market penetration: Early adoption dictated by neurology approval and formulary access.
- Reimbursement policies: Payer negotiations could lead to variations.
- Competitive pressures: The entry of generics post-patent expiry will drive downward pricing trends.
Market Penetration and Sales Volume Projections
Initial Adoption
ENLYTE SOFTGEL is expected to intermittently capture 5–10% of the partial-onset seizure market in the first two years post-launch, driven by:
- Physician familiarity with brivaracetam.
- Incremental benefits perceived in patient adherence.
- Strategic formulary placements.
Long-term Market Share
By 2028, market share could stabilize at approximately 15–20%, assuming competitive pressures and generic entrants.
Sales Volume Estimates
-
2023–2024:
Sales volume in the U.S. projected at 0.5–1 million capsules annually; corresponding revenue of approximately $15–$30 million. -
2025–2028:
Growth expected to plateau as market saturation occurs, with revenues potentially declining with the introduction of generics but maintaining a sustainable niche.
Regulatory, Patent, and IPR Impact
The patent landscape significantly influences pricing and market strategy. UCB’s primary patent estate for brivaracetam expired in 2022 [4], opening the door for generics. However, secondary patents or exclusivity periods may delay generic entry until at least 2025.
The expiration timeline, combined with patent challenges, suggests a gradual erosion of premium pricing and increased generic competition by 2025–2026.
Key Drivers of Market Success
- Formulary inclusion: Securing preferred status in insurance plans.
- Physician adoption: Educating prescribers on formulation benefits.
- Patient adherence: Market advantage via softgel administration ease.
- Pricing and reimbursement: Competitive pricing to facilitate coverage.
Conclusion and Price Projection Summary
| Year | Estimated Price per Softgel | Market Share | Approximate Sales Revenue |
|---|---|---|---|
| 2023 | $25–$30 | 5–10% | ~$15–$30 million |
| 2024 | $20–$25 | 10–15% | ~$20–$40 million |
| 2025 | $15–$20 | 15–20% | ~$25–$50 million |
| 2026–2028 | $15 | 15–20% | Stabilized around ~$30–$50 million annually |
These projections assume steady adoption, favorable formulary positioning, and eventual generic entry influencing pricing dynamics.
Key Takeaways
- Market Position: ENLYTE SOFTGEL fills a niche with patient-friendly formulation options amid a mature AED market dominated by generics.
- Pricing Dynamics: Initial premium pricing (~$25–$30 per softgel) is plausible, with a gradual decline as generic competitors emerge.
- Growth Outlook: Moderate market share growth anticipated in the medium term, constrained by patent exclusivity timelines and competitive pressures.
- Strategic Factors: Success depends heavily on formulary access, prescriber education, and reimbursement negotiations.
- Long-term Outlook: Post-generic expiry, the market is expected to consolidate around lower-priced options, with the potential for niche positioning based on formulation benefits.
FAQs
Q1: How does ENLYTE SOFTGEL compare to existing antiepileptic medications in terms of pricing?
A: Initially, ENLYTE SOFTGEL is projected to command a price similar to branded brivaracetam (around $25–$30 per softgel), higher than generics like levetiracetam. Over time, prices are expected to decrease with generic competition.
Q2: What factors will most influence the market adoption of ENLYTE SOFTGEL?
A: Key factors include formulary acceptance by payers, physician familiarity, patient preference for softgel formulations, and reimbursement policies.
Q3: When can we expect generic versions of brivaracetam to disrupt the market?
A: Patent protections may extend through 2025–2026, with potential generic entry thereafter, significantly impacting pricing and market share.
Q4: What is the potential for ENLYTE SOFTGEL to expand beyond partial-onset seizures?
A: Currently, indications are limited to adjunctive therapy for partial-onset seizures. Expansion would require additional clinical trials and regulatory approval.
Q5: How can manufacturers maintain profitability amid falling prices?
A: By securing preferred formulary status, optimizing manufacturing efficiencies, and developing niche markets based on formulation advantages and patient adherence benefits.
References
- WHO. Epilepsy Fact Sheet. World Health Organization. 2022.
- CDC. Epilepsy Data and Statistics. Centers for Disease Control and Prevention. 2021.
- Grand View Research. Epilepsy Drugs Market Size & Trends. 2022.
- U.S. Patent and Trademark Office. Patent filings related to brivaracetam. 2018–2022.
More… ↓
