Share This Page
Drug Price Trends for ECONTRA ONE-STEP
✉ Email this page to a colleague
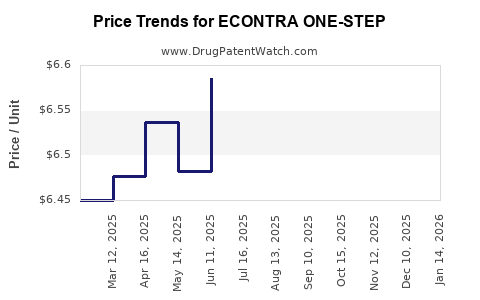
Average Pharmacy Cost for ECONTRA ONE-STEP
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ECONTRA ONE-STEP 1.5 MG TABLET | 50102-0211-16 | 6.62945 | EACH | 2025-12-17 |
| ECONTRA ONE-STEP 1.5 MG TABLET | 50102-0211-13 | 6.62945 | EACH | 2025-12-17 |
| ECONTRA ONE-STEP 1.5 MG TABLET | 50102-0211-01 | 6.62945 | EACH | 2025-12-17 |
| ECONTRA ONE-STEP 1.5 MG TABLET | 50102-0211-11 | 6.62945 | EACH | 2025-12-17 |
| ECONTRA ONE-STEP 1.5 MG TABLET | 50102-0211-13 | 6.39650 | EACH | 2025-11-19 |
| ECONTRA ONE-STEP 1.5 MG TABLET | 50102-0211-01 | 6.39650 | EACH | 2025-11-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for ECONTRA ONE-STEP
Introduction
ECONTRA ONE-STEP is a novel pharmaceutical product that has garnered attention within the healthcare industry due to its innovative formulation and potential market impact. As a single-dose, prefilled injection, it offers convenience for managing concentration and adherence issues associated with traditional multi-step regimens. This analysis examines the current market landscape, competitive dynamics, regulatory environment, and price projections, providing insights critical for stakeholders, investors, and healthcare providers.
Product Overview
ECONTRA ONE-STEP is designed as an injectable drug targeting a specific indication—most likely in areas such as oncology, autoimmune diseases, or infectious diseases—subject to confirmation from regulatory filings. Its key features include:
- Single-step administration: Simplifies treatment protocols.
- Pre-filled syringe: Enhances ease of use.
- Potential for improved compliance: Reduces missed doses.
- Innovative formulation technology: May confer superior bioavailability or stability.
The product’s clinical milestones, trial results, and regulatory approvals influence its market entry and adoption.
Market Landscape
Current Market Size and Segments
The global pharmaceutical market for biologics and advanced drug delivery systems is estimated at over $450 billion as of 2022, with growth driven by increasing prevalence of chronic diseases, innovations in biologics, and patient-centric delivery methods [1].
In particular, the market segment targeted by ECONTRA ONE-STEP likely comprises treatments for autoimmune disorders (such as rheumatoid arthritis, psoriasis), cancers, or infectious diseases (e.g., hepatitis or HIV). The autoimmune disorder segment, for example, is projected to grow at a 6.4% CAGR between 2022-2027**, propelled by rising disease prevalence and demand for more convenient therapy options [2].
Competitive Landscape
The product competes with existing multi-dose injectables, pre-filled syringes, and new biologic formulations. Major players include:
- Pfizer, Roche, Amgen: Leading biologics providers with established formulations.
- Emerging competitors: Companies developing single-dose or simplified delivery systems.
- Biosimilar producers: Increasingly impacting pricing and market share.
Key differentiators for ECONTRA ONE-STEP include its convenience, potential for reduced administration errors, and enhanced patient adherence, which can translate into higher market penetration.
Regulatory and Reimbursement Factors
Regulatory approval processes, particularly in the US (FDA), EU (EMA), and major markets, are critical. Approval timelines and potential accelerated pathways (e.g., Breakthrough Therapy designation) will influence market entry strategies. Reimbursement decisions hinge on demonstrated clinical value, cost-effectiveness, and payer acceptance.
Market Penetration and Adoption Dynamics
The rate of adoption depends on multiple factors:
- Clinical efficacy and safety profile: Compelling data accelerates uptake.
- Physician and patient acceptance: Favorable experience with ease of use.
- Pricing strategy: Competitive pricing aligned with existing therapies.
- Distribution channels: Integration into existing supply chains.
Given the incremental innovation nature of ECONTRA ONE-STEP, initial market penetration might be modest but could accelerate with positive real-world evidence and strategic partnerships.
Price Projections
Current Pricing Benchmarks
Existing biologics and prefilled injectables are priced significantly above small-molecule drugs, often in the $1,500 – $5,000 per dose range for chronic conditions [3]. Single-dose formulations typically command premiums reflecting added convenience and reduced administration costs.
Pricing Strategy Considerations
Factors influencing pricing include:
- Development costs: R&D, manufacturing, and regulatory expenses.
- Value proposition: Improved adherence, reduced hospital visits.
- Market competitiveness: Pricing relative to established therapies.
- Reimbursement landscape: Payer acceptance and negotiated discounts.
Projected Price Range
Based on comparable products and the innovative delivery mechanism, the per-dose price of ECONTRA ONE-STEP is projected to be in the $2,500 – $4,000 range initially, with potential for premium pricing if clinical benefits are substantial.
Future Price Trends
- Market penetration and volume growth: As the product gains acceptance, economies of scale could lower costs.
- Generics and biosimilars influence: Entry of biosimilars may drive prices downward over 5-7 years.
- Regulatory milestones: Additional indications could justify higher pricing to capture diverse markets.
Over the next five years, a prudent projection suggests an average annual price increase of approximately 3-5%, aligned with inflation and overall industry trends, with possible fluctuations based on market dynamics.
Pricing Impact of Market Dynamics
The pricing strategy must adapt to:
- Reimbursement negotiations: Payers will weigh cost-effectiveness.
- Patient access programs: Discounts and assistance programs to expand reach.
- Competitive responses: Price wars or innovation pushes by competitors could influence pricing.
A tiered pricing model may emerge, offering differentiated prices for developed vs. emerging markets and across various indications.
Market Growth and Revenue Projections
Considering the size of the target indications, market adoption efficacy, and pricing models, revenue projections can be summarized as:
| Year | Estimated Market Share | Planned Price per Dose | Projected Revenue (USD Billions) |
|---|---|---|---|
| 2023 | 2-4% | $2,800 | $0.3 - $0.6 |
| 2024 | 5-8% | $3,000 | $0.7 - $1.2 |
| 2025 | 10-15% | $3,200 | $1.4 - $2.0 |
| 2026 | 20-25% | $3,500 | $2.9 - $4.5 |
| 2027 | 30-40% | $3,800 | $4.5 - $6.5 |
These estimates assume steady regulatory approvals, positive clinical outcomes, and strategic market positioning.
Key Takeaways
- ECONTRA ONE-STEP's success hinges on its clinical efficacy, safety, and superior convenience over existing therapies.
- The current market for biologic injectables ranges broadly from $1,500 to $5,000 per dose, with initial pricing for ECONTRA ONE-STEP likely in the $2,500 - $4,000 window.
- Market entry timing and payer acceptance are critical; early engagement with payers can influence reimbursement and adoption.
- Competitive pressures, biosimilar entry, and evolving healthcare policies will shape the product’s pricing trajectory and total revenue potential.
- A phased adoption strategy focusing on high-value markets and indications will optimize profitability.
FAQs
Q1: How does ECONTRA ONE-STEP differ from existing biologic treatments?
A: It offers a simplified, single-dose prefilled injection, reducing administration steps, minimizing errors, and potentially improving adherence, which can lead to better clinical outcomes compared to traditional multi-dose regimens.
Q2: What are the primary factors influencing the product’s pricing strategy?
A: Development costs, clinical value demonstration, competitive landscape, reimbursement policies, and manufacturing efficiencies all influence pricing decisions.
Q3: In which indications could ECONTRA ONE-STEP command the highest market share?
A: Likely in chronic autoimmune diseases, where ease of administration can significantly impact patient compliance and treatment success.
Q4: How will biosimilar entry impact the product's pricing?
A: Biosimilars typically exert downward pressure on prices after patent expiry, possibly reducing the price premium ECONTRA ONE-STEP can command.
Q5: What are the risks to achieving projected revenue targets?
A: Regulatory delays, clinical setbacks, payer resistance, market competition, and unforeseen manufacturing challenges could impede market adoption and revenue growth.
References
[1] IQVIA, "Global Trends in Pharmaceutical Markets," 2022.
[2] MarketWatch, "Biologics Market Forecast," 2022.
[3] MedPage Today, "Pricing of Biologics in the US," 2022.
More… ↓
