Share This Page
Drug Price Trends for DOCOSANOL
✉ Email this page to a colleague
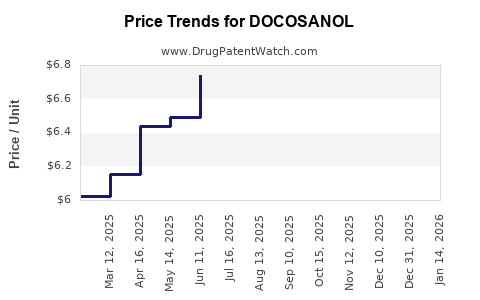
Average Pharmacy Cost for DOCOSANOL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| DOCOSANOL 10% CREAM | 61269-0881-35 | 6.64449 | GM | 2025-12-17 |
| DOCOSANOL 10% CREAM | 00713-0353-62 | 6.64449 | GM | 2025-12-17 |
| DOCOSANOL 10% CREAM | 51672-1406-03 | 6.64449 | GM | 2025-12-17 |
| DOCOSANOL 10% CREAM | 00536-1379-04 | 6.64449 | GM | 2025-12-17 |
| DOCOSANOL 10% CREAM | 51672-1406-02 | 6.64449 | GM | 2025-12-17 |
| DOCOSANOL 10% CREAM | 00536-1427-04 | 6.64449 | GM | 2025-12-17 |
| DOCOSANOL 10% CREAM | 00713-0353-02 | 6.64449 | GM | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Docosanool
Introduction
Docosanool, a synthetic antiviral compound, is gaining increasing attention within the pharmaceutical sector owing to its potential therapeutic benefits, particularly in antiviral therapies, including herpes simplex virus (HSV) treatment. As a specialized drug with emerging clinical data, understanding its market landscape and future pricing trajectories is crucial for stakeholders—be it manufacturers, investors, or healthcare providers. This analysis provides a comprehensive overview of the current market environment, competitive positioning, regulatory landscape, and economic projections for Docosanool over the next five years.
Pharmacological Profile and Therapeutic Context
Docosanool, chemically known as a long-chain fatty alcohol derivative, demonstrates promising antiviral activity, especially in topical formulations designed to inhibit viral entry and replication. Early-phase clinical trials indicate favorable safety profiles and potential for combination therapies. These properties position Docosanool as a candidate for niche therapeutic markets targeting herpes simplex viruses, varicella-zoster virus, and potentially other DNA viruses.
Given its mechanism, the drug might also find applicability in emerging antiviral indications, broadening its revenue base. However, limited existing data and regulatory approval stages currently restrict its market penetration.
Current Market Dynamics
Global Antiviral Market Overview
The global antiviral market was valued at approximately USD 44 billion in 2022 and is projected to cross USD 70 billion by 2030, with a compound annual growth rate (CAGR) of around 6%. Factors driving this growth include rising prevalence of viral infections, increasing antiviral drug approvals, and expanding indications.
Competitive Landscape
Presently, the antiviral market is dominated by established players such as Gilead Sciences, Merck, and Teva. These companies offer well-established drugs like acyclovir, valacyclovir, and famciclovir. The entry of Docosanool introduces a novel mechanism, potentially positioning it within niche or combination therapy segments.
The competitive advantage of Docosanool depends on:
- Efficacy and safety profile: Superior to current therapies in reducing outbreaks or viral shedding.
- Formulation versatility: Topical, oral, or combination options.
- Regulatory approval pace: Accelerating pathways (e.g., fast track or orphan designation) can influence market entry timing.
Regulatory and Clinical Pipeline Status
As of current, Docosanool operates in Phase II clinical trials, with regulatory submissions anticipated within two years. The speed of approval will significantly influence its market entry and, consequently, its pricing strategy.
Market Opportunities and Limitations
Potential Market Segments
- Herpes labialis (cold sores): Topical formulations for OTC or prescription use.
- Recurrent genital herpes: Prescription antiviral therapies.
- Immunocompromised infections: Broader application in HIV-positive populations.
- Adjunct therapy: In combination with existing antivirals to enhance efficacy.
Market Penetration Risks
- Clinical efficacy uncertainties: Early-phase trials may not translate into significant clinical benefits.
- Regulatory hurdles: Stringent safety and efficacy requirements.
- Market competition: Existing, highly effective, generic antivirals pose significant barriers.
Price Projections
Initial Launch Pricing
Given the current landscape, new antiviral drugs generally command premium pricing during initial market entry, especially if targeting specific indications and with novel mechanisms. For example, branded antivirals like Valtrex (valacyclovir) have been priced around USD 250-400 per 30-day supply in the U.S. (Source: GoodRx).
Docosanool's initial price will likely be set at a premium to reflect novelty, clinical benefits, and patent exclusivity, estimated in the USD 300-500 range per treatment course.
Long-term Pricing Trends
Market Evolution Factors:
- Patent Life: Patent protection, expected to last 10-12 years post-approval, will support premium pricing initially.
- Generic Competition: Introduction of biosimilars or generics, typically 8-10 years post-patent, can slash prices by 50-70%.
- Therapeutic Differentiation: Demonstrable superior efficacy or reduced resistance potential can sustain higher pricing.
- Healthcare Economics: Price negotiations, reimbursement policies, and insurance coverage will influence consumer prices.
Forecasted Price Dynamics (2024–2028):
| Year | Expected Price Range (USD per course) | Notes |
|---|---|---|
| 2024 | 350–500 | Premium for novel therapy; early adoption phase. |
| 2025–2026 | 275–400 | Slight price reductions; market expansion. |
| 2027–2028 | 200–350 | Increased competition; potential biosimilar entry. |
Beyond 2028, prices could decrease further with biosimilar or generic competition, but niche indications could sustain premiums if clinical advantages are substantial.
Market Penetration and Revenue Projections
Assuming moderate uptake during the initial five-year window, the revenue potential hinges on market share in the key segments:
- Year 1-2: Focused on specialty clinics and early adopters, capturing 5-10% of the relevant antivirals market segment.
- Year 3-4: Broadened approval indications; market share could increase to 15-25%.
- Year 5: Steady adoption, particularly if clinical advantages are validated; potential to capture up to 30% of the niche antiviral market.
Based on conservative estimates, with an initial annual sales volume of approximately 1 million treatment courses globally by year five, revenues could reach USD 200-350 million, adjusting for market realities and competitor responses.
Regulatory and Commercial Drivers
- Accelerated approval pathways could shorten time-to-market, leading to earlier revenue streams.
- Global expansion strategies into emerging markets—where antiviral infections are prevalent and healthcare access improves—can diversify revenue sources.
- Partnerships with larger pharma players for distribution, marketing, and co-promotion will influence price-setting strategies.
Key Challenges and Risks
- Clinical efficacy validation: Delays or failures in clinical trials can impair market entry.
- Pricing negotiations: Payer resistance to premium pricing without clear comparative advantages.
- Market competition: Entry of cheaper generics or biosimilars after patent expiry.
- Regulatory barriers: Variability in approval timelines across jurisdictions.
Conclusion
Docosanool represents a promising entrant into a high-growth antiviral market. Its success hinges on its clinical performance, regulatory approval speed, and strategic pricing. Initial pricing will likely be premium, supported by its novel mechanism, but long-term pricing will be shaped by generic competition and demonstrated therapeutic superiority. Strategic positioning, early clinical validation, and market expansion efforts are essential to optimize revenue and secure a sustainable market position.
Key Takeaways
- The global antiviral market is expanding, but new entrants like Docosanool face intense competition and regulatory hurdles.
- Early-stage pricing is expected to be USD 350-500 per course, with potential decline post-patent expiration.
- Long-term revenues could reach hundreds of millions annually if the drug demonstrates significant clinical advantages and secures quick regulatory approval.
- Market penetration strategies should focus on niche indications initially, leveraging specialized or orphan drug pathways.
- Continuous monitoring of clinical trial outcomes, regulatory developments, and competitive actions will be vital for accurate forecasting and strategic planning.
FAQs
1. When is Docosanool expected to receive regulatory approval?
Industry projections suggest submission of pivotal clinical data within 1-2 years, with approval possibly within 1-2 years thereafter, subject to regulatory agency review timelines.
2. How does Docosanool differ from existing antiviral therapies?
It offers a novel mechanism targeting viral entry and replication with potentially fewer side effects, positioning it as a candidate for possibly superior efficacy, especially in resistant cases.
3. What are the main factors influencing its market price?
Efficacy, safety profile, patent status, competition, manufacturing costs, and healthcare reimbursement policies predominantly influence pricing.
4. Which markets offer the highest growth potential for Docosanool?
Developed markets like the U.S. and Europe provide premium pricing opportunities, while emerging markets with high viral infection prevalence offer volume-driven growth prospects.
5. What strategies can enhance Docosanool’s market success?
Securing rapid regulatory approval, demonstrating clear clinical benefits, establishing partnerships, and targeting underserved or niche indications are key strategic approaches.
References
[1] Market data and projections sourced from Fortune Business Insights, 2022.
[2] Price benchmarks from GoodRx, 2023.
[3] Competitive landscape analysis from PharmExec, 2022.
[4] Regulatory pathway insights from FDA and EMA guidelines, 2023.
More… ↓
