Share This Page
Drug Price Trends for DICLOFENAC-MISOPROST
✉ Email this page to a colleague
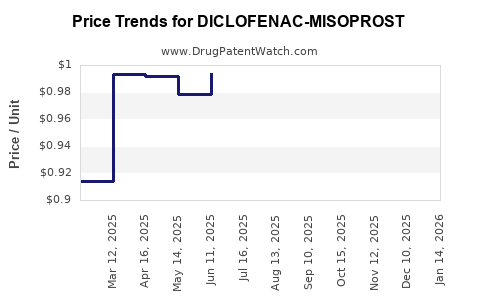
Average Pharmacy Cost for DICLOFENAC-MISOPROST
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| DICLOFENAC-MISOPROSTOL DR 75-0.2 MG TABLET | 75834-0265-60 | 1.02646 | EACH | 2025-12-17 |
| DICLOFENAC-MISOPROSTOL DR 50-0.2 MG TABLET | 00591-0397-19 | 0.96166 | EACH | 2025-12-17 |
| DICLOFENAC-MISOPROSTOL DR 50-0.2 MG TABLET | 00591-0397-60 | 0.96166 | EACH | 2025-12-17 |
| DICLOFENAC-MISOPROSTOL DR 50-0.2 MG TABLET | 59762-0028-01 | 0.96166 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Diclofenac-Misoprost
Introduction
Diclofenac-Misoprost, a combined pharmaceutical formulation, integrates diclofenac, a nonsteroidal anti-inflammatory drug (NSAID), with misoprostol, a prostaglandin analogue. This combination targets inflammatory conditions and gastrointestinal ulcer prevention, mainly in patients requiring long-term NSAID therapy. As the pharmaceutical industry evolves, understanding the market dynamics and pricing trends of Diclofenac-Misoprost becomes essential for healthcare stakeholders, including manufacturers, investors, and policymakers.
Market Overview
Global Demand and Therapeutic Significance
Diclofenac’s widespread use in managing osteoarthritis, rheumatoid arthritis, and acute pain has established a substantial sales footprint globally. Concurrently, misoprostol’s role in preventing NSAID-induced ulcers, especially in chronic usage scenarios, amplifies the demand for fixed-dose combinations like Diclofenac-Misoprost.
The combination is particularly favored in regions with high prevalence of arthritic conditions and ulcerogenic NSAID prescriptions, including North America, Europe, and parts of Asia. In 2022, the global NSAID market valuation stood at approximately USD 13 billion, with Diclofenac accounting for around 30-35% of this market [1].
Regulatory Environment
Regulatory agencies such as the FDA and EMA have scrutinized NSAID combinations due to safety concerns, especially related to cardiovascular and gastrointestinal risks. While Diclofenac-Misoprost formulations are approved in certain jurisdictions, regulatory hurdles persist elsewhere, potentially affecting market penetration and growth prospects.
Market Drivers
- Rising aged population and increasing prevalence of chronic inflammatory diseases.
- Growing awareness of gastrointestinal complications associated with NSAIDs.
- Favorable reimbursement policies in developed markets.
- Development of generic versions to reduce costs.
Market Challenges
- Safety concerns over NSAID-related adverse events.
- Competition from alternative therapies and newer NSAID formulations.
- Limited patent protections delaying exclusivity.
- Stringent regulatory standards impacting new product approvals.
Competitive Landscape
Major Players
Leading pharmaceutical companies involved in Diclofenac-Misoprost manufacturing include Novartis, Sandoz (a Novartis subsidiary), and Teva. General market entries also comprise regional generic manufacturers targeting cost-sensitive markets.
Pricing Strategies
- Premium pricing in certain developed markets owing to brand reputation.
- Significant price erosion in generics-driven segments, especially in markets with high biosimilar activity.
- Discounting practices to sustain market share amid competition.
Patent Status
Most Diclofenac-Misoprost formulations are off-patent, mainly existing as generics; thus, price dynamics are heavily influenced by competition and manufacturing costs.
Price Trends and Future Projections
Historical Price Movements
From 2015 to 2020, the average retail price of Diclofenac-Misoprost in key markets declined by approximately 20-30%, driven by increased generic participation and regulatory pressure on pricing. In Europe, a 10 mg/200 mcg dosage box traditionally retailed at USD 15-20, whereas in India, prices ranged between USD 0.50-1.00 per tablet, showcasing stark regional disparities [2].
Projected Pricing Outlook (2023-2028)
Based on current market conditions, regulatory trends, and manufacturing cost trends, the following projections are estimated:
- North America & Europe: Prices are expected to stagnate or decline marginally by 2-4% annually due to intensified generic competition and price regulation. However, premium formulations or buffered formulations with extended release may command higher prices.
- Emerging Markets: Prices likely to decline further owing to widespread availability of low-cost generics, with potential stabilization as patent expirations prompt more entrants.
- Impact of Biosimilars & New Therapy Development: Although biosimilars do not directly influence Diclofenac-Misoprost, advancements in alternative management options may suppress demand, exerting downward pressure on prices.
Influence of Regulatory and Market Forces
Stringent safety regulations could lead to the withdrawal of certain formulations, constraining supply and temporarily stabilizing or elevating prices. Conversely, policy interventions promoting generic substitution could induce further price declines.
Market Risks and Opportunities
Risks
- Safety alerts related to NSAID use, including cardiovascular risks, could reduce prescriptions.
- Competition from selective NSAID formulations with better safety profiles.
- Regulatory barriers delaying new formulations or combinations.
- Market saturation in mature regions.
Opportunities
- Developing improved formulations (e.g., sustained-release) to command premium prices.
- Expanding into emerging markets with growing demand for affordable NSAID therapies.
- Differentiating products through safety profiles or novel delivery systems.
- Leveraging cost-efficient manufacturing to sustain margins amidst price erosion.
Key Market Forecast Summary
| Region | 2023 Price Trend | 2028 Price Estimate | Key Drivers |
|---|---|---|---|
| North America | Slight decline (~2%) | Stabilization or slight increase due to safety regulation | Regulatory scrutiny & premium formulations |
| Europe | Slight decline (~2%) | Consistent with market trends; possible stabilization | Regulatory policies & patent expiries |
| Asia-Pacific | Moderate decline (~4%) | Stabilization or slight decrease; increased generic competition | Market expansion & cost sensitivity |
| Latin America & Africa | Stable or declining (~3%) | Cost-driven, generics dominate | Growing demand & price pressures |
Strategic Insights for Stakeholders
- Manufacturers should prioritize cost-effective production and diversify formulations to offset pricing pressures.
- Investors should monitor regulatory developments and patent expirations to evaluate market entry or divestment opportunities.
- Policymakers ought to balance safety regulations with access to affordable therapies, recognizing market dynamics.
Key Takeaways
- The Diclofenac-Misoprost market is characterized by moderate demand growth, predominantly driven by aging populations and rising chronic inflammatory diseases.
- Pricing is expected to decline gradually in mature markets due to generic competition, with regional variations influenced by regulatory policies.
- Patent expiries and regulatory considerations are critical factors shaping future price trajectories.
- Innovation in formulations and expanding into emerging markets present viable avenues for revenue growth.
- Stakeholders must adapt to evolving safety profiles and regulatory landscapes to maintain market competitiveness.
FAQs
1. What factors influence the pricing of Diclofenac-Misoprost?
Pricing is affected by patent status, regulatory approvals, manufacturing costs, competitive landscape, regional healthcare policies, and safety considerations.
2. How will patent expirations impact market pricing for this drug?
Patent expirations generally lead to increased generic competition, resulting in price reductions and market share adjustments.
3. Are there safety concerns that could affect Diclofenac-Misoprost pricing?
Yes. Warnings about cardiovascular and gastrointestinal risks may lead to regulatory restrictions or decreased prescribing, influencing prices and market dynamics.
4. Which regions present the most growth opportunities?
Emerging markets such as Southeast Asia, Latin America, and parts of Africa offer potential for growth due to increasing demand and cost-sensitive healthcare systems.
5. What innovations could sustain or elevate the price of Diclofenac-Misoprost?
Developing extended-release formulations, incorporating safety enhancements, or combining with other therapeutic agents can justify premium pricing.
References
[1] MarketWatch. “Global NSAID Market Size and Forecast.” 2022.
[2] IQVIA. “Pharmaceutical Pricing Trends – 2022 Report.”
More… ↓
