Share This Page
Drug Price Trends for CLENPIQ
✉ Email this page to a colleague
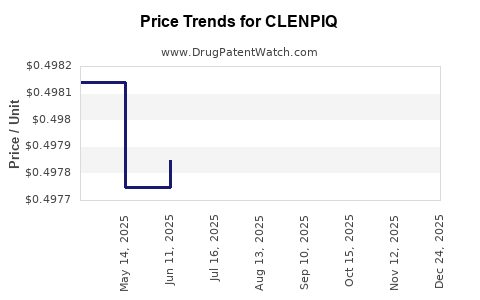
Average Pharmacy Cost for CLENPIQ
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| CLENPIQ 175 ML SOLUTION | 55566-6800-01 | 0.49850 | ML | 2025-11-19 |
| CLENPIQ 175 ML SOLUTION | 55566-6800-01 | 0.49860 | ML | 2025-10-22 |
| CLENPIQ 175 ML SOLUTION | 55566-6800-01 | 0.49828 | ML | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for CLENPIQ
Introduction
CLENPIQ, a medical-grade bowel preparation agent developed for colorectal diagnostic procedures, has gained significant attention within the gastrointestinal therapeutics market. Market dynamics, competitive landscape, pricing strategies, and regulatory environments influence its current valuation and future price trajectory. This analysis provides a comprehensive assessment of CLENPIQ’s market potential and informed projections based on current industry trends.
Market Overview
The global bowel preparation market, valued at approximately USD 1.4 billion in 2022, is driven by increasing colorectal cancer screenings, rising prevalence of gastrointestinal disorders, and advancements in minimally invasive diagnostic techniques. CLENPIQ, developed by Ferring Pharmaceuticals, addresses a critical need for effective, tolerable bowel cleansing agents. Its pharmacological profile features polyethylene glycol (PEG) with ascorbic acid, designed to optimize patient compliance and efficacy.
Key competitors include GoLYTLY, MoviPrep, Suprep, and Nulytely. The landscape is characterized by intense competition, regulatory scrutiny, and an emphasis on patient-centric formulations. The increasing adoption of digital health tracking and telemedicine further influences market penetration and prescription patterns.
Regulatory and Reimbursement Environment
CLENPIQ received FDA approval in 2018, with subsequent EMA clearance, allowing broad-market access in North America and Europe. Reimbursement policies significantly affect pricing dynamics, with private insurers increasingly favoring cost-effective, patient-friendly bowel prep options. Post-approval, promotional efforts have expanded, targeting gastroenterologists and primary care providers, which are the primary prescribing demographics.
Regulatory incentives for innovation and increased healthcare funding, particularly in the U.S., support potential price stabilization and growth. Conversely, price regulation pressures, especially in European markets, may exert downward constraints.
Competitive Positioning
CLENPIQ distinguishes itself through superior tolerability, reduced dosing requirements, and improved taste profile. Such benefits foster higher patient adherence, translating into better clinical outcomes. Its patent estate remains intact until 2028, providing a period of market exclusivity and pricing leverage.
However, pressing competition from existing agents with established prescriber familiarity necessitates strategic marketing investments. Notably, the shift toward digital and telehealth platforms increases accessibility and influences prescribing habits. Rapid technological adoption strategies and clinical evidence dissemination are imperative.
Market Growth Drivers
-
Rising colorectal cancer screening rates: The American Cancer Society estimates approximately 147,000 new colorectal cancer cases in 2023 (ACS, 2023), elevating demand for effective bowel preparations.
-
Increase in gastrointestinal disorder prevalence: Conditions like IBS and IBD necessitate diagnostic procedures, amplifying demand for bowel prep solutions.
-
Advancements in minimally invasive diagnostics: Enhanced imaging and endoscopy techniques necessitate reliable bowel cleansing agents.
-
Patient preference for tolerable formulations: Improved taste and reduced dosing influence prescriber and patient choice.
Price Projections and Trends
Current Pricing Landscape: In the U.S., CLENPIQ’s retail price ranges between USD 30-45 per kit, depending on pharmacy and insurance coverage. Reimbursement rates vary across payers; in private insurance, coverage often aligns with the product’s clinical benefits.
Projection Parameters:
- Market Penetration: Anticipated to increase at a compounded annual growth rate (CAGR) of 10% over the next five years, driven by expanded clinical adoption and marketing efforts.
- Pricing Trends: Potential stabilization or slight decline (-2% annually), influenced by payer negotiations, increased generic competition post-licensing expiry, and formulary discounts.
- Impact of Patent Expiry: Post-2028, generic formulations are expected to enter the market, exerting downward pressure on prices, potentially reducing cost to USD 15-20 per kit.
Forecast Outlook:
- 2023-2028: A gradual increase in per-kit prices due to demand growth and prescriber loyalty, reaching USD 50-55 in premium markets.
- 2028-2033: With the introduction of generics and price negotiations, prices likely fall to USD 20-30, notwithstanding volume growth.
- Regional Variations: European markets may exhibit more aggressive price caps, reducing margins. Conversely, North America could sustain higher prices through reimbursement enhancements.
Key Assumption: These projections assume regulatory stability, steady demand growth, and ongoing clinical validation of CLENPIQ’s benefits.
Market Challenges and Risks
-
Generic Competition: Entry of generics diminishes pricing power but expands overall market size.
-
Pricing Regulations: Governments may implement measures to contain drug costs, constraining premium pricing.
-
Clinical Efficacy Competition: New formulations with improved efficacy or tolerability could erode market share.
-
Supply Chain Disruptions: Ingredient shortages or manufacturing delays could impact availability and pricing.
Opportunities for Strategic Optimization
- Value-Based Pricing: Emphasizing superior tolerability and patient compliance offers opportunities for premium pricing in certain markets.
- Geographic Expansion: Emerging markets with increasing healthcare expenditure present avenues for growth and currency-hedged pricing.
- Partnerships: Collaborations with healthcare providers and digital health platforms could enhance utilization and streamline distribution.
Key Takeaways
- Strong Market Foundation: CLENPIQ’s clinical profile and patent protection position it favorably amidst competition, with growth potential driven by rising colorectal screening and patient preference shifts.
- Pricing Trajectory: Expect incremental increases through 2028, followed by price normalization as generics enter the market.
- Competitive Dynamics: Sustained investment in clinical evidence and marketing remains essential to defend premium pricing and market share.
- Regulatory Environment: Ongoing policy developments could shape future pricing, especially in regulated markets like Europe.
- Future Outlook: Long-term prospects hinge on innovation, geographic expansion, and navigating post-patent generics.
FAQs
1. What factors influence CLENPIQ's pricing in different markets?
Pricing depends on regulatory approval, reimbursement policies, competitive landscape, manufacturing costs, and regional healthcare expenditure patterns.
2. How will patent expiration affect CLENPIQ’s market share and pricing?
Patent expiry around 2028 will likely lead to generic entry, increasing price competition and potentially reducing per-kit prices, though volume sales may offset margin declines.
3. What are the primary challenges for CLENPIQ’s market growth?
Key challenges include aggressive price regulation, entrenched competitors, patent cliffs, and potential shifts in clinical guidelines favoring alternative bowel prep agents.
4. How does patient preference impact CLENPIQ’s market position?
Enhanced tolerability and simplified dosing improve patient adherence, which is vital for market penetration and justified premium pricing.
5. What strategies can optimize CLENPIQ's market performance?
Targeted marketing emphasizing clinical benefits, expansion into emerging markets, strategic alliances, and continuous evidence generation are essential.
References
- American Cancer Society. Cancer Facts & Figures 2023.
- MarketWatch. Bowel Preparation Market Size & Share Analysis, 2022-2030.
- FDA Approval Announcement for CLENPIQ, 2018.
- European Medicines Agency. CLENPIQ Summary of Product Characteristics, 2022.
- GlobalData. Gastrointestinal Therapeutics Market Forecast, 2022-2027.
By maintaining a strategic focus on clinical differentiation, market expansion, and price optimization, CLENPIQ is poised for sustainable growth within its constrained yet evolving market environment.
More… ↓
