Share This Page
Drug Price Trends for BACITRACIN-POLYMYXIN EYE OINT
✉ Email this page to a colleague
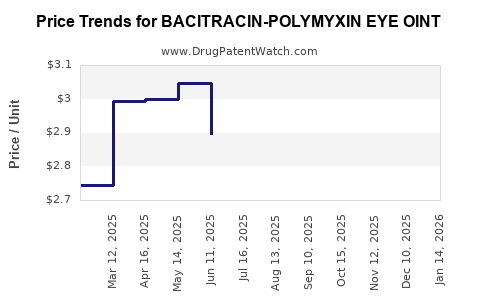
Average Pharmacy Cost for BACITRACIN-POLYMYXIN EYE OINT
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| BACITRACIN-POLYMYXIN EYE OINT | 24208-0555-55 | 3.02780 | GM | 2025-12-17 |
| BACITRACIN-POLYMYXIN EYE OINT | 24208-0555-55 | 2.96053 | GM | 2025-11-19 |
| BACITRACIN-POLYMYXIN EYE OINT | 24208-0555-55 | 2.91688 | GM | 2025-10-22 |
| BACITRACIN-POLYMYXIN EYE OINT | 24208-0555-55 | 2.77189 | GM | 2025-09-17 |
| BACITRACIN-POLYMYXIN EYE OINT | 24208-0555-55 | 2.73628 | GM | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Bacitracin-Polymyxin Eye Ointment
Introduction
Bacitracin-Polymyxin eye ointments occupy a significant niche within ophthalmic antimicrobial therapies. Combining Bacitracin, a glycopeptide antibiotic, with Polymyxin E (Colistin), a polymyxin antibiotic, these formulations address bacterial conjunctivitis and keratitis caused by susceptible strains. This analysis explores the current market landscape, competitive dynamics, regulatory environment, and projective pricing trends for Bacitracin-Polymyxin eye ointments, aiding stakeholders in strategic decision-making.
Market Overview
Therapeutic Indications and Demand Drivers
Bacitracin-Polymyxin eye ointments are primarily prescribed for bacterial ocular infections, including conjunctivitis, keratitis, and blepharitis. Rising incidences of ocular infections due to aging populations, increased contact lens misuse, and rising antibiotic resistance contribute to the demand growth. The increasing prevalence of multidrug-resistant strains necessitates effective topical antibiotics, maintaining the relevance of formulations like Bacitracin-Polymyxin.
Global Market Size
The ophthalmic antibiotics market was valued at approximately USD 1.2 billion in 2021, with a compound annual growth rate (CAGR) of about 4%, projected to reach USD 1.6 billion by 2028 [1]. While specific data on Bacitracin-Polymyxin ointments is limited, their share remains significant within combination antibiotics, with growth driven by demand for broad-spectrum agents.
Regional Market Dynamics
- North America: Dominates the market due to high healthcare spending, advanced ophthalmic care infrastructure, and established prescribing patterns favoring topical antibiotics.
- Europe: Exhibits steady growth, supported by increasing awareness of antimicrobial resistance.
- Asia-Pacific: Presents the highest potential growth owing to burgeoning healthcare infrastructure, a rising burden of ocular infections, and expanding pharmaceutical manufacturing bases. Countries like India and China lead this segment.
Competitive Landscape
Key Players
- GlaxoSmithKline (GSK): Offers combination ophthalmic antibiotics, including polymyxin-based formulations.
- Sandoz (Novartis): Supplies generic ophthalmic antibiotics, including Bacitracin-Polymyxin formulations.
- Teva Pharmaceuticals: Provides similar generic options.
- Local Generic Manufacturers: Numerous regional producers in Asia and Latin America contribute to a fragmented market.
Market Entry Barriers
- Regulatory Approvals: Stringent requirements for ophthalmic formulations, including stability, sterility, and efficacy data.
- Manufacturing Capabilities: Need for specialized sterile production facilities.
- Intellectual Property: Although many formulations are off-patent, new combination formulations or delivery systems may be patent-protected.
Regulatory Environment
- United States: FDA approval is necessary for marketed ophthalmic antibiotics; many are marketed under generic labels post patent expiry.
- Europe: EMA registration requirements apply, with national agencies overseeing approvals.
- Emerging Markets: Regulatory pathways vary, often expedited through local approvals, but quality standards remain critical.
Pricing Trends and Projections
Current Pricing
In developed markets, the retail price for Bacitracin-Polymyxin eye ointments is approximately USD 15-25 per tube (3.5g), depending on the brand and formulation. Generics dominate sales, typically priced 30-50% lower than branded products, leading to retail prices around USD 10-15.
Factors Influencing Prices
- Branding: Branded formulations maintain a premium (USD 20+).
- Regulatory Costs: High compliance costs can elevate prices for innovative formulations.
- Manufacturing Costs: Sterile manufacturing increases costs; scale can reduce per-unit expenses.
- Insurance and Reimbursement: Reimbursement policies influence out-of-pocket costs; in the U.S., coverage varies.
Price Projection (2023-2028)
- Stability in Developed Markets: Prices are expected to remain relatively stable, given patent expirations and generic competition.
- Emerging Markets: Prices may decrease due to increased generic penetration; however, local procurement policies could sustain higher prices.
- Impact of Resistance and Innovation: The introduction of novel, combination formulations with enhanced efficacy or delivery systems could command premium pricing (~USD 25-35).
Based on current market dynamics and historical trends in ophthalmic antibiotics, a modest annual price decrease of 1-2% in mature markets is anticipated for generic Bacitracin-Polymyxin ointments, with potential price stabilization or slight increases in markets with limited competition or added value formulations.
Intellectual Property and Innovation Trends
While the core formulations are largely off-patent, ongoing innovation in delivery systems (e.g., sustained-release implants) or combination enhancements could create proprietary advantages, allowing for premium pricing. Additionally, formulations targeting resistant bacterial strains or offering improved tolerability are likely to command higher prices.
Market Challenges and Opportunities
Challenges
- Antibiotic Resistance: Growing resistance reduces efficacy, potentially impacting demand.
- Regulatory Stringency: Delays and costs associated with approvals.
- Generic Competition: Intense price competition diminishes margins.
Opportunities
- Innovation in Delivery: Sustained-release or preservative-free formulations could differentiate products.
- Emerging Market Growth: Expanding healthcare access boosts demand.
- Strategic Partnerships: Collaborations for R&D and distribution can accelerate market access.
Strategic Recommendations
- Focus on Value-Added Formulations: Develop formulations with improved stability, patient comfort, or resistance profiles.
- Leverage Emerging Markets: Tailor pricing strategies to regional regulatory and economic contexts.
- Invest in R&D: Enhance efficacy against resistant strains, enabling premium pricing.
- Monitor Regulatory Changes: Stay compliant with evolving standards to minimize delays and costs.
Key Takeaways
- The global Bacitracin-Polymyxin eye ointment market remains stable, buoyed by consistent demand for broad-spectrum topical antibiotics.
- Competitive pricing pressures from generics exert downward pressure, with prices likely to decline modestly in mature markets.
- Innovation in delivery systems and formulations presents opportunities for premium pricing, especially in resistant infection scenarios.
- Emerging markets promise significant growth, driven by healthcare infrastructure expansion and rising ocular infection prevalence.
- Regulatory landscapes and antimicrobial resistance trends will substantially influence future pricing and market dynamics.
FAQs
1. Are Bacitracin-Polymyxin eye ointments under patent protection?
Most formulations are off-patent, leading to widespread generic availability and competitive pricing.
2. What factors most influence the pricing of Bacitracin-Polymyxin eye ointments?
Manufacturing costs, branding, regulatory expenses, market competition, and regional reimbursement policies primarily determine pricing.
3. How does antimicrobial resistance impact the market for this drug?
Rising resistance can decrease efficacy, prompting demand for newer formulations and possibly increasing prices if innovation occurs.
4. What are the key regulatory hurdles for market expansion?
Ensuring safety, efficacy, and sterility through rigorous clinical and manufacturing standards can delay approvals and increase costs.
5. What strategic opportunities exist for new entrants?
Innovating with sustained-release formats, preservative-free options, or formulations targeting resistant strains offers differentiation and premium pricing potential.
References
[1] Grand View Research. (2022). Ophthalmic Antibiotics Market Size, Share & Trends.
[2] MarketsandMarkets. (2021). Ophthalmic Drugs Market by Application, Route of Administration, and Region - Global Forecast to 2028.
More… ↓
