Share This Page
Drug Price Trends for ADAPALENE-BNZYL PEROX
✉ Email this page to a colleague
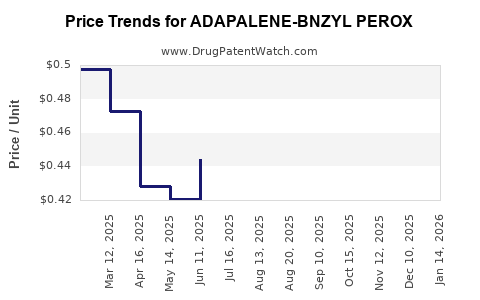
Average Pharmacy Cost for ADAPALENE-BNZYL PEROX
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ADAPALENE-BNZYL PEROX 0.3-2.5% | 72578-0119-04 | 0.60897 | GM | 2025-12-17 |
| ADAPALENE-BNZYL PEROX 0.1-2.5% | 21922-0052-50 | 0.35185 | GM | 2025-12-17 |
| ADAPALENE-BNZYL PEROX 0.1-2.5% | 45802-0846-01 | 0.35185 | GM | 2025-12-17 |
| ADAPALENE-BNZYL PEROX 0.1-2.5% | 68462-0301-47 | 0.35185 | GM | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for ADAPALENE-BNZYL PEROXIDE
Introduction
Adapalene Benzoyl Peroxide (ADAPALENE-BNZYL PEROX) combines a topical retinoid with an antimicrobial agent, primarily used to treat moderate to severe acne vulgaris. The formulation merges adapalene’s comedolytic properties with benzoyl peroxide's antibacterial effects, offering a comprehensive approach to acne management. As dermatology remains a lucrative sector within the pharmaceutical landscape, understanding market trends and price dynamics for this combination therapy is vital for stakeholders, including manufacturers, investors, and healthcare providers.
Market Overview
Global Acne Therapeutics Market
The global acne dermatology market was valued at approximately USD 4.5 billion in 2022, with an expected CAGR of 6% from 2023 to 2030 [1]. The rising prevalence of acne, especially among adolescents and young adults, coupled with increasing awareness and accessibility to topical treatments, sustains market growth. The demand for combination therapies like adapalene-benzoyl peroxide formulations is escalating due to their improved efficacy and reduced side effect profiles compared to monotherapies.
Key Players and Patent Landscape
Leading pharmaceutical companies, such as Galderma (marketed as Differin), Almirall, and major generics producers, dominate the market through various formulations. Galderma holds significant market share with its patented formulations of adapalene-benzoyl peroxide gels, with key patents expiring between 2024 and 2026, opening the door for generics.
Patent expirations critically influence market dynamics, licensing opportunities, and price competition. For instance, the expiration of the Differin gel patent in 2024 could lead to increased generic penetration, intensifying price competition.
Regulatory and Geographic Factors
Regulatory approvals in key markets like the US, EU, and Asia-Pacific shape the product launch timelines and market penetration. The FDA approved the first adapalene-benzoyl peroxide combination in 2016, with subsequent approvals in Europe and Asia. The preference for prescription versus OTC status varies by region, impacting access and pricing.
Market Drivers and Restraints
Drivers
- Rising Acne Prevalence: Acne affects over 85% of adolescents and a significant portion of adults, fostering consistent demand for effective topical treatments [2].
- Combination Efficacy: Studies reveal superior outcomes with combination therapies versus monotherapy, driving clinician preference [3].
- Product Innovation: Advancements in formulation technology, including gels, creams, and wipes, enhance patient adherence.
- Expanded Regulation: Lengthy patent protections maintain premium pricing, though impending expirations threaten price declines.
Restraints
- Generic Competition: Post-patent expiry, generic versions significantly reduce prices.
- Side Effect Profile: Common adverse effects, such as skin irritation, may limit broader acceptance.
- Pricing Regulations: Some markets impose price caps on dermatological drugs, constraining profit margins.
Price Trends and Projections
Historical Pricing Analysis
Currently, the average wholesale price (AWP) for branded adapalene-benzoyl peroxide gels (e.g., Differin Gel 0.1%/2.5%) in the US ranges between USD 200-300 per 30 grams[4]. Generics are priced approximately 50-70% lower, around USD 100-150 for equivalent quantities.
In Europe, retail prices tend to be comparable, influenced by regional healthcare policies and reimbursement systems. In the absence of patents, generic formulations dominate, leading to a notable decrease in prices over the past three years.
Projected Price Trajectories
- Short-term (1-2 years): With patent protections still in place, branded product prices are projected to remain stable or slightly increase due to inflation and supply chain factors. Expect prices in the USD 210-330 range per 30 grams.
- Medium-term (3-5 years): As patent expirations occur—anticipated between 2024 and 2026—market entry of generics is likely. Prices for generics may decline by 40-60%, settling around USD 60-90 per 30 grams.
- Long-term (5+ years): Market saturation with multiple generic manufacturers could further reduce prices, possibly stabilizing around USD 50 or lower, depending on regional policies and manufacturing costs.
Regional variations will significantly influence these projections. For example, in emerging markets with less regulation, prices could be even lower, whereas in markets with stringent reimbursement, prices might remain elevated for longer.
Market Penetration and Revenue Forecasting
Current Market Penetration
Adapalene-benzoyl peroxide products are estimated to hold approximately 20-30% of the overall acne treatment market, owing to their clinical efficacy. The volume sales are driven primarily by prescription prescriptions, although OTC availability exists in certain regions.
Future Revenue Potential
Post-patent expiry, increased penetration by generics is expected to boost unit volume sales, although unit price decline will temper revenue growth. Industry models suggest a compound annual growth rate (CAGR) of 4-5% in unit sales over the next decade, with revenue growth plateauing or slightly decreasing due to price erosion.
Regulatory and Competitive Considerations
Regulatory pathways for generics involve abbreviated new drug applications (ANDAs), emphasizing bioequivalence. Market entrants should anticipate delays associated with regulatory submissions, manufacturing scale-up, and market access strategies.
The rise of novel delivery systems—such as foam formulations or film strips—may also introduce competition, potentially affecting pricing strategies and market share.
Key Challenges and Opportunities
-
Challenges:
- Patent cliffs leading to aggressive price competition.
- Market saturation in mature regions.
- Regulatory variability across jurisdictions.
-
Opportunities:
- Development of enhanced formulations with improved patient adherence.
- Entry into emerging markets with less price sensitivity.
- Strategic licensing agreements with patent holders.
Conclusion
The market for adapalene-benzoyl peroxide combination therapy is poised for significant transformation driven by patent expiry cycles and evolving competitive landscapes. While branded products command premium pricing, impending patent expirations will likely precipitate a sharp decline in prices following generic entry. Stakeholders should prepare for intensified price competition in the medium term, capitalizing on innovation, regulatory strategies, and market expansion efforts to sustain revenues.
Key Takeaways
- The global acne treatment market is expanding, with adapalene-benzoyl peroxide formulations occupying a prominent position.
- Patent expiration from 2024 to 2026 is set to stimulate generic competition, markedly reducing prices.
- Current prices for branded formulations are USD 200-300 per 30 grams; generics could fall below USD 100 within 2-3 years post-patent expiry.
- Regional regulatory frameworks and healthcare policies heavily influence pricing trajectories.
- Innovation in formulation and delivery mechanisms remains critical for maintaining competitive advantage amid impending price erosion.
FAQs
1. When will generic versions of adapalene-benzoyl peroxide become widely available?
Patent expirations are anticipated between 2024 and 2026, after which generic manufacturers are expected to launch comparable products, aligning with regulatory approval timelines.
2. How will patent expiries affect the pricing of adapalene-benzoyl peroxide products?
Post-expiry, increased generic competition generally leads to significant price reductions, potentially decreasing prices by up to 60-70%, depending on regional market dynamics and manufacturing costs.
3. Are there any regional differences in the market for adapalene-benzoyl peroxide?
Yes. Developed markets like the US and EU tend to have higher prices and regulatory standards, while emerging markets may see lower prices due to less regulatory stringency and greater price sensitivity.
4. What role does formulation innovation play in this market?
Innovations such as foam or dissolvable strips can improve patient adherence, differentiate products, and sustain premium pricing even amid generic competition.
5. What strategic actions should manufacturers consider given upcoming generic competition?
They should focus on patent strategies, diversify formulations, expand into emerging markets, optimize manufacturing efficiencies, and explore licensing arrangements to maintain profitability.
References
[1] MarketsandMarkets. (2022). Acne vulgaris therapeutics market by product, distribution channel, and region.
[2] Zaenglein AL, et al. (2016). "Guidelines of care for acne vulgaris," Journal of the American Academy of Dermatology.
[3] Thiboutot D, et al. (2018). "Combination therapies for acne," Dermatologic Therapy.
[4] GoodRx. (2023). Average wholesale prices for topical acne treatments.
More… ↓
