Pregabalin - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for pregabalin and what is the scope of patent protection?
Pregabalin
is the generic ingredient in three branded drugs marketed by Upjohn, ACI, Actavis Elizabeth, Adaptis, Alembic, Alkem Labs Ltd, Amneal Pharms Co, Apotex, Aurobindo Pharma, Cadila Pharms Ltd, Changzhou Pharm, Chartwell Rx, Cipla, Creekwood Pharms, Dr Reddys, Eskayef, Hetero Labs Ltd Iii, Invagen Pharms, Lupin Ltd, MSN, Mylan, Prinston Inc, Renata, Rising, Sciegen Pharms Inc, Strides Pharma, Sun Pharm, Teva Pharms, Yiling, Zydus Pharms, Patrin, Alvogen, Epic Pharma Llc, and Rubicon, and is included in forty-seven NDAs. There are three patents protecting this compound and six Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Pregabalin has thirty-seven patent family members in thirty-three countries.
There are forty-one drug master file entries for pregabalin. Fifty-one suppliers are listed for this compound. There are nine tentative approvals for this compound.
Summary for pregabalin
| International Patents: | 37 |
| US Patents: | 3 |
| Tradenames: | 3 |
| Applicants: | 34 |
| NDAs: | 47 |
| Drug Master File Entries: | 41 |
| Finished Product Suppliers / Packagers: | 51 |
| Raw Ingredient (Bulk) Api Vendors: | 101 |
| Clinical Trials: | 516 |
| Patent Applications: | 6,331 |
| Formulation / Manufacturing: | see details |
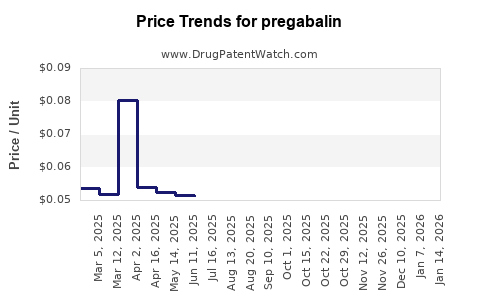
| Drug Prices: | Drug price trends for pregabalin |
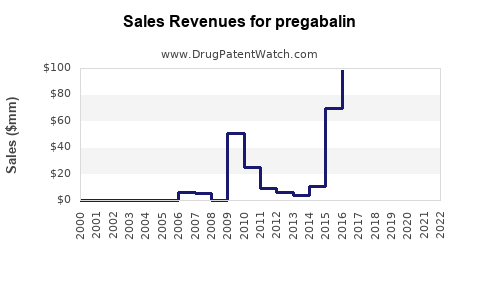
| Drug Sales Revenues: | Drug sales revenues for pregabalin |
| What excipients (inactive ingredients) are in pregabalin? | pregabalin excipients list |
| DailyMed Link: | pregabalin at DailyMed |
Recent Clinical Trials for pregabalin
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Istanbul Physical Medicine Rehabilitation Training and Research Hospital | N/A |
| Egymedicalpedia | Early Phase 1 |
| Beijing Tiantan Hospital | N/A |
Generic filers with tentative approvals for PREGABALIN
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 100MG | CAPSULE;ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | 75MG | CAPSULE;ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | 50MG | CAPSULE;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Medical Subject Heading (MeSH) Categories for pregabalin
Paragraph IV (Patent) Challenges for PREGABALIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LYRICA CR | Extended-release Tablets | pregabalin | 82.5 mg and 165 mg | 209501 | 1 | 2018-02-02 |
| LYRICA CR | Extended-release Tablets | pregabalin | 330 mg | 209501 | 1 | 2018-01-29 |
| LYRICA | Oral Solution | pregabalin | 20 mg/mL | 022488 | 1 | 2010-05-19 |
| LYRICA | Capsules | pregabalin | 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg and 300 mg | 021446 | 8 | 2008-12-30 |
US Patents and Regulatory Information for pregabalin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurobindo Pharma | PREGABALIN | pregabalin | CAPSULE;ORAL | 205321-001 | Mar 29, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Zydus Pharms | PREGABALIN | pregabalin | CAPSULE;ORAL | 206752-004 | Dec 9, 2022 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms Co | PREGABALIN | pregabalin | CAPSULE;ORAL | 209743-001 | Jul 19, 2019 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Renata | PREGABALIN | pregabalin | CAPSULE;ORAL | 210585-006 | Dec 26, 2019 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Aurobindo Pharma | PREGABALIN | pregabalin | CAPSULE;ORAL | 205321-007 | Mar 29, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for pregabalin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | LYRICA | pregabalin | SOLUTION;ORAL | 022488-001 | Jan 4, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LYRICA | pregabalin | CAPSULE;ORAL | 021446-007 | Dec 30, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LYRICA | pregabalin | CAPSULE;ORAL | 021446-003 | Dec 30, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LYRICA | pregabalin | CAPSULE;ORAL | 021446-004 | Dec 30, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LYRICA | pregabalin | CAPSULE;ORAL | 021446-001 | Dec 30, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for pregabalin
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Zentiva k.s. | Pregabalin Zentiva k.s. | pregabalin | EMEA/H/C/004277 Neuropathic painPregabalin Zentiva k.s. is indicated for the treatment of peripheral and central neuropathic pain in adults.EpilepsyPregabalin Zentiva k.s. is indicated as adjunctive therapy in adults with partial seizures with or without secondary generalisation.Generalised anxiety disorderPregabalin Zentiva k.s. is indicated for the treatment of generalised anxiety disorder (GAD) in adults. |
Withdrawn | yes | no | no | 2017-02-27 | |
| Zentiva, k.s. | Pregabalin Zentiva | pregabalin | EMEA/H/C/003900 Neuropathic pain, , , Pregabalin Zentiva is indicated for the treatment of peripheral and central neuropathic pain in adults., , , Epilepsy, , , Pregabalin Zentiva is indicated as adjunctive therapy in adults with partial seizures with or without secondary generalisation., , , Generalised anxiety disorder, , , Pregabalin Zentiva is indicated for the treatment of generalised anxiety disorder (GAD) in adults., , |
Authorised | yes | no | no | 2015-07-17 | |
| Mylan S.A.S. | Pregabalin Mylan Pharma | pregabalin | EMEA/H/C/003962 EpilepsyPregabalin Mylan Pharma is indicated as adjunctive therapy in adults with partial seizures with or without secondary generalisation.Generalised Anxiety DisorderPregabalin Mylan Pharma is indicated for the treatment of Generalised Anxiety Disorder (GAD) in adults. |
Withdrawn | yes | no | no | 2015-06-25 | |
| Upjohn EESV | Pregabalin Pfizer | pregabalin | EMEA/H/C/003880 Neuropathic painPregabalin Pfizer is indicated for the treatment of peripheral and central neuropathic pain in adults.EpilepsyPregabalin Pfizer is indicated as adjunctive therapy in adults with partial seizures with or without secondary generalisation.Generalised Anxiety DisorderPregabalin Pfizer is indicated for the treatment of Generalised Anxiety Disorder (GAD) in adults. |
Authorised | no | no | no | 2014-04-10 | |
| Upjohn EESV | Lyrica | pregabalin | EMEA/H/C/000546 Neuropathic painLyrica is indicated for the treatment of peripheral and central neuropathic pain in adults.EpilepsyLyrica is indicated as adjunctive therapy in adults with partial seizures with or without secondary generalisation.Generalised anxiety disorderLyrica is indicated for the treatment of generalised anxiety disorder (GAD) in adults. |
Authorised | no | no | no | 2004-07-05 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for pregabalin
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Morocco | 30135 | COMPOSITIONS PHARMACEUTIQUES SOLIDES CONTENANT DE LA PREGABALINE | ⤷ Try a Trial |
| Taiwan | 200803831 | Solid pharmaceutical compositions containing pregabalin | ⤷ Try a Trial |
| South Africa | 200803115 | Solid pharmaceutical compositions containing pregabalin | ⤷ Try a Trial |
| Taiwan | I330080 | ⤷ Try a Trial | |
| Argentina | 058175 | COMPOSICIONES FARMACEUTICAS SOLIDAS QUE CONTIENEN PREGABALINA | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for pregabalin
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0641330 | SPC/GB04/034 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PREGABALIN (S-(+)-4-AMINO-3(2-METHYLPROPYL)BUTANOIC ACID) OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACTIVE SALT.; REGISTERED: UK EU/1/04/279/001 20040706; UK EU/1/04/279/002 20040706; UK EU/1/04/279/003 20040706; UK EU/1/04/279/004 20040706; UK EU/1/04/279/005 20040706; UK EU/1/04/279/006 20040706; UK EU/1/04/279/025 20040706; UK EU/1/04/279/019 20040706; UK EU/1/04/279/020 20040706; UK EU/1/04/279/021 20040706; UK EU/1/04/279/022 20040706; UK EU/1/04/279/023 20040706; UK EU/1/04/279/024 20040706; UK EU/1/04/279/013 20040706; UK EU/1/04/279/014 20040706; UK EU/1/04/279/015 20040706; UK EU/1/04/279/016 20040706; UK EU/1/04/279/017 20040706; UK EU/1/04/279/018 20040706; UK EU/ |
| 0934061 | PA2004017 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PREGABALINUM ((S)-3-(AMINOMETIL)-5-METILHEKSANO RûGðTIS) |
| 0641330 | 27/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: PREGABALIN, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES; REGISTRATION NO/DATE: EU/1/04/279/001 - EU/1/04/279/025 20040706 |
| 0641330 | C300164 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: PREGABALINE, DESGEWENST IN DE; REGISTRATION NO/DATE: EU/1/04/279/001 20040706 |
| 0934061 | PA2004017,C0934061 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PREGABALINUM ((S)-3-(AMINOMETIL)-5-METILHEKSANO RUGSTIS); REGISTRATION NO/DATE: EU/1/04/279/001-025 20040725 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |