Sun Pharm Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SUN PHARM, and what generic alternatives to SUN PHARM drugs are available?
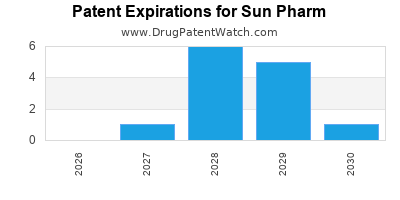
SUN PHARM has five hundred and forty-eight approved drugs.
There are thirty-three US patents protecting SUN PHARM drugs. There are eighteen tentative approvals on SUN PHARM drugs.
There are three hundred and seventy-nine patent family members on SUN PHARM drugs in fifty-eight countries and six hundred and nineteen supplementary protection certificates in nineteen countries.
Summary for Sun Pharm
| International Patents: | 379 |
| US Patents: | 33 |
| Tradenames: | 354 |
| Ingredients: | 328 |
| NDAs: | 548 |
| Patent Litigation for Sun Pharm: | See patent lawsuits for Sun Pharm |
| PTAB Cases with Sun Pharm as petitioner: | See PTAB cases with Sun Pharm as petitioner |
Drugs and US Patents for Sun Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun Pharm Inds Ltd | ATORVASTATIN CALCIUM | atorvastatin calcium | TABLET;ORAL | 076477-004 | Nov 30, 2011 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Inds Ltd | TOPIRAMATE | topiramate | TABLET;ORAL | 076327-001 | Mar 27, 2009 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sun Pharm Industries | PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE | amitriptyline hydrochloride; perphenazine | TABLET;ORAL | 071079-001 | Nov 12, 1986 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sun Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sun Pharm Industries | BACTRIM DS | sulfamethoxazole; trimethoprim | TABLET;ORAL | 017377-002 | Approved Prior to Jan 1, 1982 | RE28636 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-006 | Aug 15, 2014 | 9,078,925 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-006 | Aug 15, 2014 | 8,952,064 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SUN PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-01-07 |
| ➤ Subscribe | Capsules | 35 mg | ➤ Subscribe | 2015-11-25 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Capsules | 30 mg and 40 mg | ➤ Subscribe | 2012-12-31 |
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-06-19 |
| ➤ Subscribe | Capsules | 25 mg | ➤ Subscribe | 2016-05-16 |
| ➤ Subscribe | Tablets | 125 mg | ➤ Subscribe | 2018-07-23 |
International Patents for Sun Pharm Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2963206 | ⤷ Try a Trial |
| Spain | 2462946 | ⤷ Try a Trial |
| China | 102159570 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sun Pharm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2139494 | 122020000043 | Germany | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN UND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160715 |
| 0521471 | SPC/GB03/033 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ROSUVASTATIN OPTIONALLY IN THE FORM OF A NON-TOXIC PHARMACEUTICALLY ACCEPTABLE SALT, PARTICULARLY THE CALCIUM SALT.; REGISTERED: NL 26872 20021106; NL 26873 20021106; NL 26874 20021106; UK PL 17901/0201 20030321; UK PL 17901/0202 20030321; UK PL 17901/0203 20030321 |
| 2435025 | 2019C/532 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE GLYCOPYRROLATE (INCLUANT LES SELS ACCEPTABLES PHARMACEUTIQUEMENT, LES ESTERS, LES ENANTIOMERES OU LES AUTRES DERIVES DE CECI) ET DE FORMOTEROL (INCLUANT LES SELS ACCEPTABLES PHARMACEUTIQUEMENT, LES ESTERS, LES ENANTIOMERES OU LES AUTRES DERIVES DE CECI); AUTHORISATION NUMBER AND DATE: EU/1/18/1339 20181220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.