Cipla Company Profile
✉ Email this page to a colleague
What is the competitive landscape for CIPLA, and when can generic versions of CIPLA drugs launch?
CIPLA has ninety-two approved drugs.
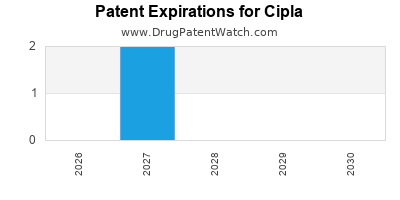
There are six US patents protecting CIPLA drugs. There are thirty-three tentative approvals on CIPLA drugs.
There are thirty patent family members on CIPLA drugs in twenty-one countries and four hundred and thirty-nine supplementary protection certificates in seventeen countries.
Summary for Cipla
| International Patents: | 30 |
| US Patents: | 6 |
| Tradenames: | 81 |
| Ingredients: | 81 |
| NDAs: | 92 |
| Patent Litigation for Cipla: | See patent lawsuits for Cipla |
| PTAB Cases with Cipla as petitioner: | See PTAB cases with Cipla as petitioner |
Drugs and US Patents for Cipla
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cipla | DEFERASIROX | deferasirox | GRANULE;ORAL | 215026-002 | Feb 23, 2022 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla | LENALIDOMIDE | lenalidomide | CAPSULE;ORAL | 210435-002 | Sep 6, 2022 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla | EMTRICITABINE | emtricitabine | CAPSULE;ORAL | 091168-001 | Jul 2, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla Usa | ZEMDRI | plazomicin sulfate | SOLUTION;INTRAVENOUS | 210303-001 | Jun 25, 2018 | RX | Yes | Yes | 8,822,424 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cipla | RANOLAZINE | ranolazine | TABLET, EXTENDED RELEASE;ORAL | 211291-002 | May 28, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Cipla | CELECOXIB | celecoxib | CAPSULE;ORAL | 207446-002 | Sep 23, 2015 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla | CINACALCET HYDROCHLORIDE | cinacalcet hydrochloride | TABLET;ORAL | 208915-003 | Mar 8, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Cipla Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Israel | 205880 | ⤷ Try a Trial |
| Slovenia | 2217610 | ⤷ Try a Trial |
| European Patent Office | 2217610 | ⤷ Try a Trial |
| China | 103360440 | ⤷ Try a Trial |
| European Patent Office | 2083823 | ⤷ Try a Trial |
| Canada | 2706369 | ⤷ Try a Trial |
| Hungary | E030523 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Cipla Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2924034 | 1990024-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: DORAVIRINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN COMBINATION WITH LAMIVUDINE AND TENOFOVIR DISOPROXIL FUMARATE; REG. NO/DATE: EU/1/18/1333 20181126 |
| 2137537 | 122014000069 | Germany | ⤷ Try a Trial | PRODUCT NAME: DIMETHYLFUMARAT; REGISTRATION NO/DATE: EU/1/13/837/001-002 20140130 |
| 0382526 | 19675032 | Germany | ⤷ Try a Trial | PRODUCT NAME: LAMIVUDINE, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH GEEIGNETEN SALZES; NAT. REGISTRATION NO/DATE: EU/1/96/015/001-002 19960808 FIRST REGISTRATION: CH 53662 53663 19960228 |
| 0443983 | 2007C/043 | Belgium | ⤷ Try a Trial | PRODUCT NAME: AMLODIPINE ET VALSARTAN; NATL. REGISTRATION NO/DATE: EU/1/06/370/001 20070118; FIRST REGISTRATION: CH 57771 20061222 |
| 2666774 | LUC00167 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: RELEBACTAM, EVENTUELLEMENT SOUS FORME DE MONOHYDRATE, IMIPENEME ET CILASTATINE, EVENTUELLEMENT SOUS FORME DE SEL DE SODIUM; AUTHORISATION NUMBER AND DATE: EU/1/19/1420 20200217 |
| 2203431 | 92666 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OU UN SEL QUI EN DERIVE, Y COMPRIS DASABUVIR SODIUMMONOHYDRATE. FIRST REGISTRATION: 20150119 |
| 2487166 | PA2016038 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ELVITEGRAVIRAS + KOBICISTATAS + EMTRICITABINAS + TENOFOVIRALAFENAMIDAS; REGISTRATION NO/DATE: EU/1/15/1061 20151119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |