Estradiol - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for estradiol and what is the scope of freedom to operate?
Estradiol
is the generic ingredient in forty-three branded drugs marketed by Allergan, Alvogen, Mylan, Padagis Israel, Prasco, Teva Pharms Usa, Abbvie, Bayer Hlthcare, Amneal, Mylan Technologies, Zydus Pharms, Parke Davis, Noven, Mylan Speciality Lp, Ascend Theraps Us, Vertical Pharms, Chemo Research Sl, Novitium Pharma, Pfizer, Mayne Pharma, Padagis Us, Women First Hlthcare, Novartis, Ortho Mcneil Pharm, Sandoz, Bristol Myers Squibb, Barr Labs Inc, Dr Reddys Labs Sa, Epic Pharma Llc, Lannett Co Inc, Usl Pharma, Duramed Pharms Barr, Novo Nordisk Inc, Amneal Pharms, Glenmark Pharms Ltd, Millicent, Apil, Dr Reddys, Pharmacia And Upjohn, Watson Labs, Exeltis Usa Inc, Par Sterile Products, Am Regent, Fosun Pharma, Hikma, Xiromed, Savage Labs, Noven Pharms Inc, Lupin Ltd, Barr, Breckenridge Pharm, Mylan Labs Ltd, Naari Pte Ltd, Novast Labs, Aurobindo Pharma Ltd, Myovant Sciences, and Teva Womens, and is included in eighty-four NDAs. There are fifty-five patents protecting this compound and four Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Estradiol has one hundred and eighty-three patent family members in twenty-six countries.
There are seventy-five drug master file entries for estradiol. Forty-five suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for estradiol
| International Patents: | 183 |
| US Patents: | 55 |
| Tradenames: | 43 |
| Applicants: | 57 |
| NDAs: | 84 |
| Drug Master File Entries: | 75 |
| Finished Product Suppliers / Packagers: | 45 |
| Raw Ingredient (Bulk) Api Vendors: | 109 |
| Clinical Trials: | 987 |
| Patent Applications: | 6,728 |
| Formulation / Manufacturing: | see details |
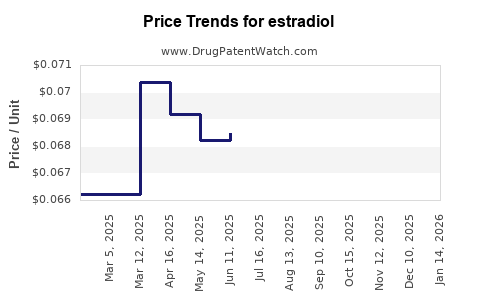
| Drug Prices: | Drug price trends for estradiol |
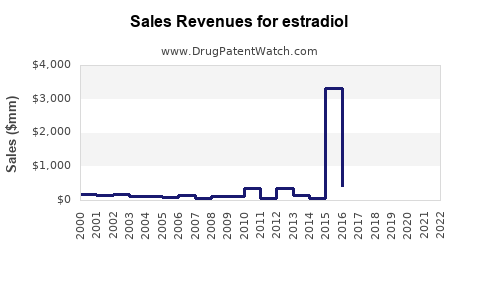
| Drug Sales Revenues: | Drug sales revenues for estradiol |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for estradiol |
| What excipients (inactive ingredients) are in estradiol? | estradiol excipients list |
| DailyMed Link: | estradiol at DailyMed |
Recent Clinical Trials for estradiol
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Gary Schwartz | Phase 1 |
| Dartmouth-Hitchcock Medical Center | Phase 1 |
| Beni-Suef University | Phase 2/Phase 3 |
Generic filers with tentative approvals for ESTRADIOL
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 1MG;2MG;2MG | TABLET;ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | 3MG;2MG;3MG | TABLET;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for estradiol
| Drug Class | Estrogen |
| Mechanism of Action | Estrogen Receptor Agonists |
Medical Subject Heading (MeSH) Categories for estradiol
Paragraph IV (Patent) Challenges for ESTRADIOL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| IMVEXXY | Vaginal Inserts | estradiol | 4 mcg and 10 mcg | 208564 | 1 | 2019-12-30 |
| MINIVELLE | Transdermal System | estradiol | 0.025 mg/day | 203752 | 1 | 2015-05-08 |
| MINIVELLE | Transdermal System | estradiol | 0.0375 mg/day 0.05 mg/day 0.075 mg/day 0.1 mg/day | 203752 | 1 | 2014-08-18 |
| VAGIFEM | Vaginal Tablets | estradiol | 10 mcg | 020908 | 1 | 2013-01-02 |
| VIVELLE-DOT | Transdermal System | estradiol | 0.025 mg/day 0.0375 mg/day 0.05 mg/days 0.075 mg/day 0.1 mg/day | 020538 | 1 | 2010-04-27 |
| CLIMARA | Transdermal System | estradiol | 0.05 mg/day and 0.1 mg/day | 020375 | 2005-09-12 |
US Patents and Regulatory Information for estradiol
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lupin Ltd | AMABELZ | estradiol; norethindrone acetate | TABLET;ORAL | 203339-001 | Jun 20, 2016 | BX | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Mayne Pharma | BIJUVA | estradiol; progesterone | CAPSULE;ORAL | 210132-002 | Dec 28, 2021 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | AB3 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Usl Pharma | ESTRADIOL | estradiol | TABLET;ORAL | 040297-001 | Apr 17, 2002 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Xiromed | ESTRADIOL VALERATE | estradiol valerate | INJECTABLE;INJECTION | 216656-003 | Apr 28, 2023 | AO | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for estradiol
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sandoz | VIVELLE | estradiol | SYSTEM;TRANSDERMAL | 020323-004 | Oct 28, 1994 | ⤷ Try a Trial | ⤷ Try a Trial |
| Pfizer | ESTRING | estradiol | INSERT, EXTENDED RELEASE;VAGINAL | 020472-001 | Apr 26, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sandoz | VIVELLE | estradiol | SYSTEM;TRANSDERMAL | 020323-001 | Oct 28, 1994 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sandoz | VIVELLE | estradiol | SYSTEM;TRANSDERMAL | 020323-005 | Aug 16, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-003 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for estradiol
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2013192248 | ⤷ Try a Trial | |
| Australia | 2017208300 | Soluble Estradiol Capsule For Vaginal Insertion | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2015148952 | ⤷ Try a Trial | |
| Australia | 2015237243 | Progesterone formulations | ⤷ Try a Trial |
| Portugal | 2782584 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for estradiol
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0398460 | 12/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON IN KOMBINATION MIT ESTRADIOL; NAT. REGISTRATION NO/DATE: 1-25178, 1-25179 20031127; FIRST REGISTRATION: NL RVG 27505 20021211 |
| 0770388 | 09C0018 | France | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL VALERATE; DIENOGEST; NAT. REGISTRATION NO/DATE: NL35170 20081210; FIRST REGISTRATION: BE327792 20081103 |
| 0770388 | PA2009004 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLI VALERAS + DIENOGESTUM; NAT. REGISTRATION NO/DATE: LT/1/09/1512/001, 2009 04 06 LT/1/09/1512/002, 2009 04 06 LT/1/09/1512/003 20090406; FIRST REGISTRATION: BE 327792 20081103 |
| 2782584 | 132021000000197 | Italy | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLO (17SS-ESTRADIOLO) IN PARTICOLARE NELLA FORMA EMIIDRATA, E PROGESTERONE COMPRENDENTI LE VARIE FORME DI ESTRADIOLO (17SS-ESTRADIOLO), QUALI LE FORME IDRATE E SOLVATATE, INCLUDENDO LA FORMA EMIIDRATA, ED I SUOI SALI.(BIJUVA); AUTHORISATION NUMBER(S) AND DATE(S): BE582231, 20210406;048335018 -048335020, 20210517 |
| 0398460 | SPC/GB04/032 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL, OPTIONALLY IN THE FORM OF A HYDRATE, TOGETHER WITH DROSPIRENONE; REGISTERED: NL RVG 27505 20021211; UK PL 00053/0341 20040310 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.