BUPRENORPHINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Buprenorphine, and what generic alternatives are available?
Buprenorphine is a drug marketed by Alvogen, Amneal, Aveva, Mylan Tech Viatris, Watson Labs Teva, Am Regent, Hikma, Hospira, Par Sterile Products, Actavis Elizabeth, Barr, Ethypharm, Mylan, Rhodes Pharms, Rubicon, Sun Pharm, Dr Reddys Labs Sa, Mylan Technologies, Alkem Labs Ltd, Amneal Pharms, Ethypharm Usa Corp, Lannett Co Inc, Specgx Llc, Teva Pharms Usa, and Wes Pharma Inc. and is included in thirty-six NDAs.
The generic ingredient in BUPRENORPHINE is buprenorphine hydrochloride; naloxone hydrochloride. There are twenty-nine drug master file entries for this compound. Twenty-four suppliers are listed for this compound. Additional details are available on the buprenorphine hydrochloride; naloxone hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Buprenorphine
A generic version of BUPRENORPHINE was approved as buprenorphine hydrochloride; naloxone hydrochloride by ACTAVIS ELIZABETH on February 22nd, 2013.
Summary for BUPRENORPHINE
| US Patents: | 0 |
| Applicants: | 25 |
| NDAs: | 36 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 19 |
| Clinical Trials: | 490 |
| Patent Applications: | 4,004 |
| Formulation / Manufacturing: | see details |
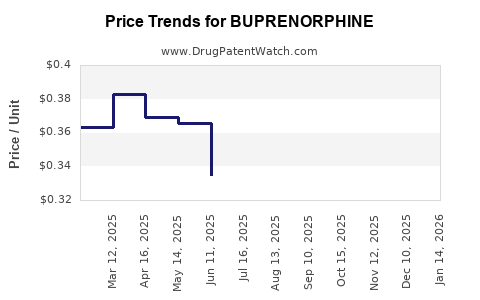
| Drug Prices: | Drug price information for BUPRENORPHINE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BUPRENORPHINE |
| What excipients (inactive ingredients) are in BUPRENORPHINE? | BUPRENORPHINE excipients list |
| DailyMed Link: | BUPRENORPHINE at DailyMed |
Recent Clinical Trials for BUPRENORPHINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Gangnam Severance Hospital | N/A |
| Washington University School of Medicine | Phase 3 |
| Loyola University | Phase 4 |
Pharmacology for BUPRENORPHINE
| Drug Class | Partial Opioid Agonist |
| Mechanism of Action | Partial Opioid Agonists |
Medical Subject Heading (MeSH) Categories for BUPRENORPHINE
Anatomical Therapeutic Chemical (ATC) Classes for BUPRENORPHINE
Paragraph IV (Patent) Challenges for BUPRENORPHINE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BUTRANS | Transdermal System | buprenorphine | 15 mcg/hr | 021306 | 1 | 2013-12-16 |
| BUTRANS | Transdermal System | buprenorphine | 5 mcg/hr 10 mcg/hr 20 mcg/hr | 021306 | 1 | 2013-06-06 |
US Patents and Regulatory Information for BUPRENORPHINE
EU/EMA Drug Approvals for BUPRENORPHINE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| L. Molteni & C. dei Fratelli Alitti Società di Esercizio S.p.A. | Sixmo | buprenorphine | EMEA/H/C/004743 Sixmo is indicated for substitution treatment for opioid dependence in clinically stable adult patients who require no more than 8 mg/day of sublingual buprenorphine, within a framework of medical, social and psychological treatment. |
Authorised | no | no | no | 2019-06-19 | |
| Camurus AB | Buvidal | buprenorphine | EMEA/H/C/004651 Treatment of opioid dependence within a framework of medical, social and psychological treatment. Treatment is intended for use in adults and adolescents aged 16 years or over. |
Authorised | no | no | no | 2018-11-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


