DROSPIRENONE - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for drospirenone and what is the scope of freedom to operate?
Drospirenone
is the generic ingredient in twenty branded drugs marketed by Exeltis Usa Inc, Mayne Pharma, Bayer Hlthcare, Barr, Glenmark Pharms Ltd, Hetero Labs, Hlthcare, Jubilant Cadista, Mylan Labs Ltd, Watson Labs, Sun Pharm, Aurobindo Pharma Ltd, Xiromed, Novast Labs, Lupin Ltd, Apotex, Dr Reddys Labs Sa, Naari Pte Ltd, and Watson Labs Inc, and is included in thirty-seven NDAs. There are eighteen patents protecting this compound. Additional information is available in the individual branded drug profile pages.Drospirenone has sixty-five patent family members in twenty-nine countries.
There are eleven drug master file entries for drospirenone. One supplier is listed for this compound. There is one tentative approval for this compound.
Summary for DROSPIRENONE
| International Patents: | 65 |
| US Patents: | 18 |
| Tradenames: | 20 |
| Applicants: | 19 |
| NDAs: | 37 |
| Drug Master File Entries: | 11 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 98 |
| Patent Applications: | 3,354 |
| Formulation / Manufacturing: | see details |
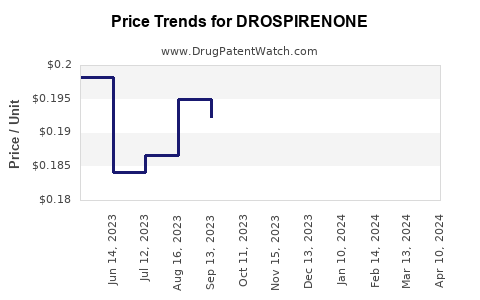
| Drug Prices: | Drug price trends for DROSPIRENONE |
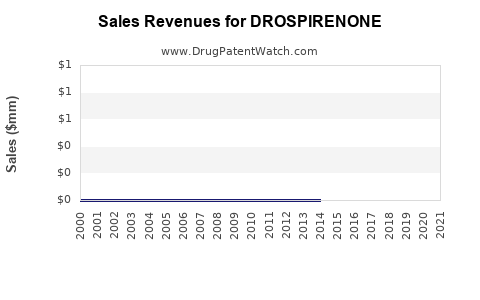
| Drug Sales Revenues: | Drug sales revenues for DROSPIRENONE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for DROSPIRENONE |
| What excipients (inactive ingredients) are in DROSPIRENONE? | DROSPIRENONE excipients list |
| DailyMed Link: | DROSPIRENONE at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DROSPIRENONE
Generic Entry Date for DROSPIRENONE*:
Constraining patent/regulatory exclusivity:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DROSPIRENONE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Colorado, Denver | Phase 2 |
| Janssen Pharmaceutica N.V., Belgium | Phase 1 |
| Bristol Myers Squibb Company (BMS) | Phase 1 |
Generic filers with tentative approvals for DROSPIRENONE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 4MG | TABLET;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Paragraph IV (Patent) Challenges for DROSPIRENONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SLYND | Tablets | drospirenone | 4 mg | 211367 | 1 | 2022-01-07 |
US Patents and Regulatory Information for DROSPIRENONE
International Patents for DROSPIRENONE
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 2020015 | ⤷ Try a Trial | |
| Slovenia | 3632448 | ⤷ Try a Trial | |
| South Korea | 102539030 | ⤷ Try a Trial | |
| Peru | 20161410 | COMPOSICION FARMACEUTICA QUE COMPRENDE DROSPIRENONA Y KIT ANTICONCEPTIVO | ⤷ Try a Trial |
| Chile | 2012003685 | Kit farmaceutico que comprende una o mas unidades de envasado que comprenden 21 a 28 unidades de dosificacion activas diarias en que cada una comprende por lo menos 2 mg de drosperidona, sin estrogernos; uso del kit y de una composicion farmaceutica que comprende drosperidona para preparar un medicamento util como anticonceptivo. | ⤷ Try a Trial |
| Guatemala | 201200336 | COMPOSICIÒN FARMACÈUTICA QUE COMPRENDE DROSPIRENONA Y KIT ANTICONCEPTIVO | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DROSPIRENONE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2588114 | 122021000065 | Germany | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; NAT. REGISTRATION NO/DATE: 7002248.00.00 20210426; FIRST REGISTRATION: DK 61678 20191016 |
| 3632448 | CA 2022 00016 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; NAT. REG. NO/DATE: 61678 20191016; FIRST REG. NO/DATE: DK 61678 20191016 |
| 2588114 | 2020C/518 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; AUTHORISATION NUMBER AND DATE: BE548284 20191107 |
| 1380301 | 2009C/007 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 3632448 | C202230031 | Spain | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONA; NATIONAL AUTHORISATION NUMBER: 84603-SE/H/1869/001/DC; DATE OF AUTHORISATION: 20191025; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SE/H/1809/001/DC; DATE OF FIRST AUTHORISATION IN EEA: 20191016 |
| 3632448 | LUC00266 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE; AUTHORISATION NUMBER AND DATE: 61678, 20210401 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |