Lupin Ltd Company Profile
✉ Email this page to a colleague
What is the competitive landscape for LUPIN LTD, and what generic alternatives to LUPIN LTD drugs are available?
LUPIN LTD has one hundred and fifty-eight approved drugs.
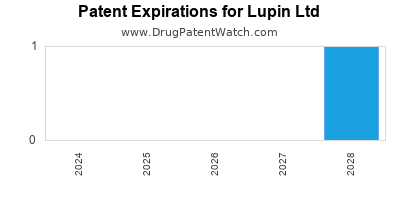
There is one US patent protecting LUPIN LTD drugs. There are nineteen tentative approvals on LUPIN LTD drugs.
There are two patent family members on LUPIN LTD drugs in two countries and five hundred and eighty-three supplementary protection certificates in seventeen countries.
Summary for Lupin Ltd
| International Patents: | 2 |
| US Patents: | 1 |
| Tradenames: | 137 |
| Ingredients: | 120 |
| NDAs: | 158 |
| Drug Master File Entries: | 194 |
| Patent Litigation for Lupin Ltd: | See patent lawsuits for Lupin Ltd |
| PTAB Cases with Lupin Ltd as petitioner: | See PTAB cases with Lupin Ltd as petitioner |
Drugs and US Patents for Lupin Ltd
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lupin Ltd | LORAZEPAM | lorazepam | CONCENTRATE;ORAL | 091407-001 | Feb 19, 2013 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | DIVALPROEX SODIUM | divalproex sodium | TABLET, EXTENDED RELEASE;ORAL | 209286-001 | Oct 18, 2019 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | QUETIAPINE FUMARATE | quetiapine fumarate | TABLET;ORAL | 201109-003 | Mar 27, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | OLMESARTAN MEDOXOMIL | olmesartan medoxomil | TABLET;ORAL | 206631-003 | Apr 27, 2017 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Lupin Ltd | IRBESARTAN | irbesartan | TABLET;ORAL | 201531-003 | Oct 15, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | LAMIVUDINE | lamivudine | TABLET;ORAL | 205217-001 | Dec 18, 2014 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for LUPIN LTD drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Injection | 250 mcg/0.5 mL, 1 mL PFS | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | for Oral Suspension | 500 mg/5 mL | ➤ Subscribe | 2014-07-22 |
International Patents for Lupin Ltd Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2007119249 | ⤷ Try a Trial |
| Germany | 112007000920 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Lupin Ltd Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0733366 | 98C0030 | Belgium | ⤷ Try a Trial | PRODUCT NAME: KALII LOSARTAN, HYDROCHLOROTHIAZIDUM; NAT. REGISTRATION NO/DATE: 922 IS 174 F 3 19980223; FIRST REGISTRATION: FR 338 520.7 19950215 |
| 1441735 | 08C0026 | France | ⤷ Try a Trial | PRODUCT NAME: RALTEGRAVIR POTASSIUM; REGISTRATION NO/DATE: EU/1/07/436/001 20080102 |
| 1392714 | 2017/019 | Ireland | ⤷ Try a Trial | PRODUCT NAME: OBETICHOLIC ACID; REGISTRATION NO/DATE: EU/1/16/1139/001 EU/1/16/1139/002 20161212 |
| 2498758 | CA 2020 00017 | Denmark | ⤷ Try a Trial | PRODUCT NAME: METFORMIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; SAXAGLIPTIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; DAPAGLIFLOZIN ELLER ET FARMACEUTISK ACCEPTABELT SOLVAT DERAF; REG. NO/DATE: EU/1/19/1401 20191113 |
| 1663240 | 132016000024787 | Italy | ⤷ Try a Trial | PRODUCT NAME: ASSOCIAZIONE DI RILPIVIRINA E OGNI SUA FORMA TERAPEUTICAMENTE EQUIVALENTE PROTETTA DAL BREVETTO DI BASE, COME SALI DI ADDIZIONE FARMACEUTICAMENTE ACCETTABILI DI RILPIVIRINA, COMPRESO IL SUO SALE CLORIDRATO, TENOFOVIR, IN PARTICOLARE TENOFOVIR DISOPROXIL FUMARATO E EMITRICITABINA(EVIPLERA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/11/737/001-002, 20111128 |
| 1534313 | C 2015 055 | Romania | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE DE FENILEFRINA SAU O SARE ACCEPTABILAFARMACEUTIC A ACESTEIA SI KETOROLAC SATIONAL AUTHORISATION: 20150728; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/1018; DATE OF FIRST AUTHORISATION IN EEA: 20150728 U O SARE ACCEPTABILA FARMACEUTIC AACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/15/1018; DATE OF NA |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.