Sun Pharm Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SUN PHARM, and what generic alternatives to SUN PHARM drugs are available?
SUN PHARM has five hundred and forty-eight approved drugs.
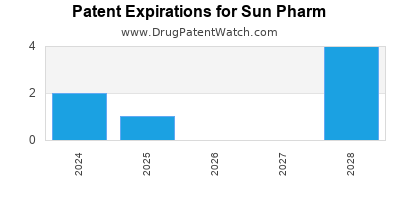
There are thirty-three US patents protecting SUN PHARM drugs. There are eighteen tentative approvals on SUN PHARM drugs.
There are three hundred and seventy-nine patent family members on SUN PHARM drugs in fifty-eight countries and six hundred and nineteen supplementary protection certificates in nineteen countries.
Summary for Sun Pharm
| International Patents: | 379 |
| US Patents: | 33 |
| Tradenames: | 354 |
| Ingredients: | 328 |
| NDAs: | 548 |
| Patent Litigation for Sun Pharm: | See patent lawsuits for Sun Pharm |
| PTAB Cases with Sun Pharm as petitioner: | See PTAB cases with Sun Pharm as petitioner |
Drugs and US Patents for Sun Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun Pharm Inds Ltd | LISINOPRIL | lisinopril | TABLET;ORAL | 075944-004 | Jul 1, 2002 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm | ABSORICA LD | isotretinoin | CAPSULE;ORAL | 211913-002 | Nov 5, 2019 | RX | Yes | No | 9,700,535 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Sun Pharm | RIVASTIGMINE TARTRATE | rivastigmine tartrate | CAPSULE;ORAL | 077131-004 | Oct 22, 2007 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Inds | FEXOFENADINE HYDROCHLORIDE ALLERGY | fexofenadine hydrochloride | TABLET;ORAL | 091567-004 | Feb 6, 2012 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sun Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-005 | Aug 15, 2014 | 8,952,064 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-001 | May 25, 2012 | 9,089,534 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-004 | May 25, 2012 | 9,078,925 | ⤷ Try a Trial |
| Sun Pharm Industries | BACTRIM | sulfamethoxazole; trimethoprim | TABLET;ORAL | 017377-001 | Approved Prior to Jan 1, 1982 | RE28636 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SUN PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-06-19 |
| ➤ Subscribe | Capsules | 25 mg | ➤ Subscribe | 2016-05-16 |
| ➤ Subscribe | Tablets | 125 mg | ➤ Subscribe | 2018-07-23 |
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-01-07 |
| ➤ Subscribe | Capsules | 35 mg | ➤ Subscribe | 2015-11-25 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Capsules | 30 mg and 40 mg | ➤ Subscribe | 2012-12-31 |
International Patents for Sun Pharm Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| New Zealand | 584555 | ⤷ Try a Trial |
| South Korea | 101495192 | ⤷ Try a Trial |
| Eurasian Patent Organization | 018302 | ⤷ Try a Trial |
| Canada | 2956902 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sun Pharm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0281459 | 98C0036 | France | ⤷ Try a Trial | PRODUCT NAME: CLOPIDOGREL HYDROGENE SULFATE; REGISTRATION NO/DATE IN FRANCE: EU/1/98 /069/001 DU 19980715; REGISTRATION NO/DATE AT EEC: DU EU/1-/98/069/001 |
| 0398460 | C300221 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON EN ETHINYLESTRADIOL; REGISTRATION NO/DATE: RVG 23827 20000307 |
| 1255752 | 122008000002 | Germany | ⤷ Try a Trial | PRODUCT NAME: SUNITINIB, GEGEBENENFALLS IN DER FORM EINES PHARMAZEUTISCH VERTRAEGLICHEN SALZES, EINSCHLIESSLICH DES L-MALATSALZES; REGISTRATION NO/DATE: EU/1/06/347/001-003 20060719 |
| 1586316 | C 2011 004 | Romania | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC, SARURILE SI HIDRATII SAI ACCEPTABILI FARMACEUTICACID 2-[2-AMINO-3-(4-BROMOBENZOIL)FENILACETIC; NATIONAL AUTHORISATION NUMBER: RO EU/1/11/692/001; DATE OF NATIONAL AUTHORISATION: 20110518; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/11/692/001; DATE OF FIRST AUTHORISATION IN EEA: 20110518 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.