Terbinafine - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for terbinafine and what is the scope of patent protection?
Terbinafine
is the generic ingredient in three branded drugs marketed by Karo Hlthcare, Novartis, Taro, Aurobindo Pharma, Breckenridge Pharm, Chartwell, Cipla, Dr Reddys Labs Inc, Emed Medcl, Gedeon Richter Usa, Glenmark Generics, Heritage Pharma Avet, Invagen Pharms, Mylan, Orbion Pharms, Roxane, and Wockhardt, and is included in twenty-four NDAs. Additional information is available in the individual branded drug profile pages.There are twenty-seven drug master file entries for terbinafine. There are four tentative approvals for this compound.
Summary for terbinafine
| US Patents: | 0 |
| Tradenames: | 3 |
| Applicants: | 17 |
| NDAs: | 24 |
| Drug Master File Entries: | 27 |
| Raw Ingredient (Bulk) Api Vendors: | 91 |
| Clinical Trials: | 45 |
| Patent Applications: | 7,520 |
| Formulation / Manufacturing: | see details |
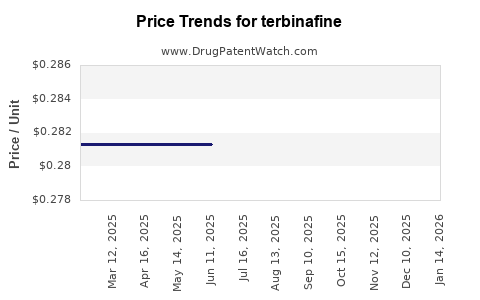
| Drug Prices: | Drug price trends for terbinafine |
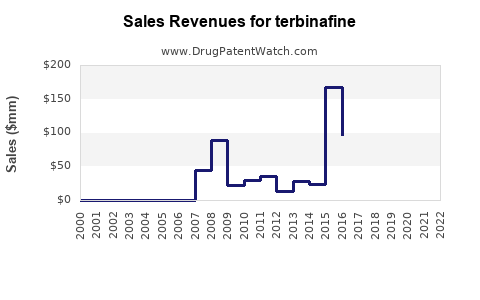
| Drug Sales Revenues: | Drug sales revenues for terbinafine |
| What excipients (inactive ingredients) are in terbinafine? | terbinafine excipients list |
| DailyMed Link: | terbinafine at DailyMed |
Recent Clinical Trials for terbinafine
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dhaka Medical College | Phase 2/Phase 3 |
| Moberg Pharma AB | Phase 3 |
| IQVIA Biotech | Phase 3 |
Generic filers with tentative approvals for TERBINAFINE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 250MG BASE | TABLET; ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 250MG BASE | TABLET;ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 250MG BASE | TABLET;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
US Patents and Regulatory Information for terbinafine
Expired US Patents for terbinafine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Karo Hlthcare | LAMISIL AT | terbinafine | GEL;TOPICAL | 021958-001 | Jul 24, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Karo Hlthcare | LAMISIL AT | terbinafine | GEL;TOPICAL | 021958-001 | Jul 24, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Karo Hlthcare | LAMISIL | terbinafine | GEL;TOPICAL | 020846-001 | Apr 29, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Karo Hlthcare | LAMISIL | terbinafine | GEL;TOPICAL | 020846-001 | Apr 29, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Karo Hlthcare | LAMISIL | terbinafine | GEL;TOPICAL | 020846-001 | Apr 29, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Karo Hlthcare | LAMISIL AT | terbinafine | GEL;TOPICAL | 021958-001 | Jul 24, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |