Taro Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TARO, and what generic alternatives to TARO drugs are available?
TARO has two hundred and fifty-three approved drugs.
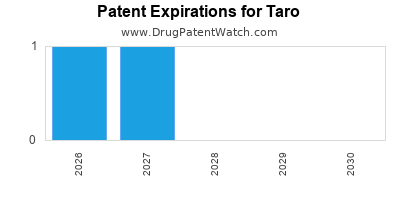
There are eight US patents protecting TARO drugs. There are four tentative approvals on TARO drugs.
There are thirty-one patent family members on TARO drugs in twelve countries and one hundred and seventy-three supplementary protection certificates in fourteen countries.
Summary for Taro
| International Patents: | 31 |
| US Patents: | 8 |
| Tradenames: | 133 |
| Ingredients: | 116 |
| NDAs: | 253 |
| Patent Litigation for Taro: | See patent lawsuits for Taro |
| PTAB Cases with Taro as petitioner: | See PTAB cases with Taro as petitioner |
Drugs and US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | IVERMECTIN | ivermectin | LOTION;TOPICAL | 210720-001 | May 6, 2020 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | ACETIC ACID | acetic acid, glacial | SOLUTION/DROPS;OTIC | 088638-001 | Sep 6, 1984 | AT | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro Pharm Inds | ETODOLAC | etodolac | TABLET;ORAL | 075074-002 | Apr 25, 2000 | AB | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro Pharm Inds | CARBAMAZEPINE | carbamazepine | TABLET, CHEWABLE;ORAL | 075687-002 | Jul 29, 2002 | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | WARFARIN SODIUM | warfarin sodium | TABLET;ORAL | 040301-004 | Jul 15, 1999 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,656,482 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 5,881,926 | ⤷ Try a Trial |
| Taro | TOPICORT | desoximetasone | SPRAY;TOPICAL | 204141-001 | Apr 11, 2013 | 5,990,100 | ⤷ Try a Trial |
| Taro | PLIAGLIS | lidocaine; tetracaine | CREAM;TOPICAL | 021717-001 | Jun 29, 2006 | 5,919,479 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,071,523 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TARO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Spray | 0.25% | ➤ Subscribe | 2013-12-18 |
| ➤ Subscribe | Topical Lotion | 0.5% | ➤ Subscribe | 2011-03-16 |
International Patents for Taro Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 102834096 | ⤷ Try a Trial |
| New Zealand | 566117 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2011088333 | ⤷ Try a Trial |
| Canada | 2822220 | ⤷ Try a Trial |
| South Korea | 20080044245 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Taro Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1458369 | 132008901685368 | Italy | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE E BENZOILE PEROSSIDO(EPIDUO); AUTHORISATION NUMBER(S) AND DATE(S): 40440, 20071218;DA 038261018/M A 038261057/M, 20080618 |
| 3347352 | 2290051-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: LENACAPAVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR LENACAPAVIR SODIUM; REG. NO/DATE: EU/1/22/1671 20220819 |
| 1586316 | 122011100019 | Germany | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC (2-AMINO-3-(4-BROMOBENZOYL)PHENYLESSIGSAEURE); REGISTRATION NO/DATE: EU/1/11/692/001 20110518 |
| 2506844 | 18C1022 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON COMPRENANT UN SEL PHARMACEUTIQUEMENT ACCEPTABLE D'UMECLIDINIUM (EN PARTICULIER LE BROMURE D'UMECLIDINIUM), DU VILANTEROL OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (EN PARTICULIER LE TRIFENATATE DE VILANTEROL), ET DU FUROATE DE FLUTICASONE; REGISTRATION NO/DATE: EU/1/17/1236 20171117 |
| 1856135 | CA 2020 00018 | Denmark | ⤷ Try a Trial | PRODUCT NAME: FOSTAMATINIB OR A PHARMACEUTICALLY ACCEPTABLE SALT OF FOSTAMATINIB, OR A HYDRATE, SOLVATE OR N-OXIDE OF FOSTAMATINIB OR THE PHARMACEUTICALLY ACCEPTABLE SALT OF FOSTAMATINIB, ESPECIALLY FOSTAMATINIB DISODIUM, OPTIONALLY IN FORM OF A HYDRATE; REG. NO/DATE: EU/1/19/1405 20200113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.