XERAVA Drug Patent Profile
✉ Email this page to a colleague
When do Xerava patents expire, and what generic alternatives are available?
Xerava is a drug marketed by Tetraphase Pharms and is included in one NDA. There are four patents protecting this drug.
This drug has eighty patent family members in thirty-three countries.
The generic ingredient in XERAVA is eravacycline dihydrochloride. One supplier is listed for this compound. Additional details are available on the eravacycline dihydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Xerava
Xerava was eligible for patent challenges on August 27, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 19, 2037. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for XERAVA
| International Patents: | 80 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 27 |
| Clinical Trials: | 1 |
| Patent Applications: | 120 |
| Drug Prices: | Drug price information for XERAVA |
| What excipients (inactive ingredients) are in XERAVA? | XERAVA excipients list |
| DailyMed Link: | XERAVA at DailyMed |
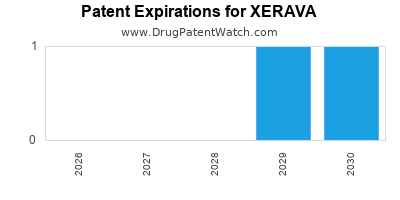

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for XERAVA
Generic Entry Date for XERAVA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for XERAVA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| West Virginia University | Phase 2 |
Pharmacology for XERAVA
| Drug Class | Tetracycline-class Antibacterial |
Anatomical Therapeutic Chemical (ATC) Classes for XERAVA
US Patents and Regulatory Information for XERAVA
XERAVA is protected by four US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of XERAVA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting XERAVA
Crystalline forms of eravacycline
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
C7-fluoro substituted tetracycline compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF COMPLICATED INTRA-ABDOMINAL INFECTIONS IN PATIENTS 18 YEARS OF AGE AND OLDER
C7-fluoro substituted tetracycline compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting XERAVA
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
GENERATING ANTIBIOTIC INCENTIVES NOW
Exclusivity Expiration: ⤷ Try a Trial
International Patents for XERAVA
When does loss-of-exclusivity occur for XERAVA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
China
Patent: 0582486
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 29236
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 19531321
Estimated Expiration: ⤷ Try a Trial
Patent: 22186979
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 567
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 019500822
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201903327P
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 190065414
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering XERAVA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| New Zealand | 603568 | C7-fluoro substituted tetracycline compounds | ⤷ Try a Trial |
| Canada | 2732883 | COMPOSES DE TETRACYCLINE C7-FLUOROSUBSTITUEE (C7-FLUORO SUBSTITUTED TETRACYCLINE COMPOUNDS) | ⤷ Try a Trial |
| Israel | 211120 | תרכובת, טטראציקלין מותמרת בעמדה 7c עם פלואור, תכשיר רוקחות המכיל אותה ושימוש בה להכנת תרופות לטיפול בזיהום הנגרם על ידי בקטריה (C7-fluoro substituted tetracycline compound, pharmaceutical composition comprising it and its use in the manufacture of medicaments for treating infection caused by bacteria) | ⤷ Try a Trial |
| Serbia | 54485 | C7-FLUORO SUPSTITUISANA TETRACIKLINSKA JEDINJENJA (C7-FLUORO SUBSTITUTED TETRACYCLINE COMPOUNDS) | ⤷ Try a Trial |
| South Korea | 20110058800 | C7-FLUORO SUBSTITUTED TETRACYCLINE COMPOUNDS | ⤷ Try a Trial |
| Hong Kong | 1193412 | -氟取代的四環素化合物 (C7-FLUORO SUBSTITUTED TETRACYCLINE COMPOUNDS C7-) | ⤷ Try a Trial |
| South Korea | 101679023 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for XERAVA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2323972 | CA 2019 00009 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ERAVACYCLIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/18/1312 20180209 |
| 2323972 | SPC/GB19/017 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ERAVACYCLINE OR PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK PLGB 24626/0005 20180924; UK PLGB 24626/0006 20180924; UK EU/1/18/1312 (NI) 20180924 |
| 2323972 | C20190009 00277 | Estonia | ⤷ Try a Trial | PRODUCT NAME: ERAVATSUEKLIIN;REG NO/DATE: EU/1/18/1312 24.09.2018 |
| 2323972 | CR 2019 00009 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ERAVACYCLIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/18/1312 20180209 |
| 2323972 | LUC00107 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ERAVACYCLINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/18/1312 20180924 |
| 2323972 | 19C1012 | France | ⤷ Try a Trial | PRODUCT NAME: ERAVACYCLINE OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/18/1312 20180924 |
| 2323972 | 715 | Finland | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


